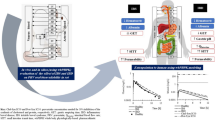
Abstract
Targeting drugs to the inflamed intestinal tissue(s) represents a major advancement in the treatment of inflammatory bowel disease (IBD). In this work we present a powerful in-silico modeling approach to guide the molecular design of novel prodrugs targeting the enzyme PLA2, which is overexpressed in the inflamed tissues of IBD patients. The prodrug consists of the drug moiety bound to the sn-2 position of phospholipid (PL) through a carbonic linker, aiming to allow PLA2 to release the free drug. The linker length dictates the affinity of the PL-drug conjugate to PLA2, and the optimal linker will enable maximal PLA2-mediated activation. Thermodynamic integration and Weighted Histogram Analysis Method (WHAM)/Umbrella Sampling method were used to compute the changes in PLA2 transition state binding free energy of the prodrug molecule (∆∆Gtr) associated with decreasing/increasing linker length. The simulations revealed that 6-carbons linker is the optimal one, whereas shorter or longer linkers resulted in decreased PLA2-mediated activation. These in-silico results were shown to be in excellent correlation with experimental in-vitro data. Overall, this modern computational approach enables optimization of the molecular design of novel prodrugs, which may allow targeting the free drug specifically to the diseased intestinal tissue of IBD patients.






Similar content being viewed by others
References
Dahan A, Zimmermann EM, Ben-Shabat S (2014) Molecules 19(10):16489
Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Jarvinen T, Savolainen J (2008) Nat Rev Drug Discov 7(3):255
Yu LS (1981) J Clin Pharm Ther 6(4):287
Ettmayer P, Amidon GL, Clement B, Testa B (2004) J Med Chem 47(10):2393
Sun J, Dahan A, Amidon GL (2010) J Med Chem 53(2):624
Sun J, Dahan A, Walls ZF, Lai L, Lee KD, Amidon GL (2010) Mol Pharm 7(6):2362
Bai JPF, Hu M, Subramanian P, Mosberg HI, Amidon GL (1992) J Pharm Sci 81(2):113
Dahan A, Wolk O, Yang P, Mittal S, Wu Z, Landowski CP, Amidon GL (2014) Mol Pharm 11(12):4385
Fleisher D, Stewart BH, Amidon GL (1985) Methods Enzymol 112:360
Banerjee PK, Amidon GL (1985) Design of prodrugs. Elsevier, New York, p 93
Han H, de Vrueh RL, Rhie JK, Covitz KM, Smith PL, Lee CP, Oh DM, Sadee W, Amidon GL (1998) Pharm Res 15(8):1154
Sabit H, Dahan A, Sun J, Provoda CJ, Lee K-D, Hilfinger JH, Amidon GL (2013) Mol Pharm 10(4):1417
Dahan A, Khamis M, Agbaria R, Karaman R (2012) Expert Opin Drug Deliv 9(8):1001
Han HK, Amidon GL (2000) AAPS PharmSci 2(1): E6
Hoult JR (1986) Drugs 32(Suppl 1):18
Stella VJ, Himmelstein KJ (1980) J Med Chem 23(12):1275
Kesisoglou F, Zimmermann EM (2005) Expert Opin Drug Deliv 2(3):451
Dahan A, Amidon GL, Zimmermann EM (2010) Expert Rev Clin Immunol 6(4):543
Biasi F, Leonarduzzi G, Oteiza PI, Poli G (2013) Antioxid Redox Signal 19(14):1711
Wolk O, Epstein S, Ioffe-Dahan V, Ben-Shabat S, Dahan A (2013) Expert Opin Drug Deliv 10(9):1275
Kurz M, Scriba GK (2000) Chem Phys Lipids 107(2):143
Haapamäki MM, Grönroos JM, Nurmi H, Alanen K, Kallajoki M, Nevalainen TJ (1997) Gut 40(1):95
Lilja I, Smedh K, Olaison G, Sjödahl R, Tagesson C, Gustafson-Svärd C (1995) Gut 37(3):380
Minami T, Tojo H, Shinomura Y, Matsuzawa Y, Okamoto M (1994) Gut 35(11):1593
Haapamaki MM, Gronroos JM, Nurmi H, Irjala K, Alanen KA, Nevalainen TJ (1999) Scand J Clin Lab Invest 59(4):279
Andresen TL, Jensen SS, Kaasgaard T, Jorgensen K (2005) Curr Drug Deliv 2(4):353
Hansen AH, Mouritsen OG, Arouri A (2015) Int J Pharm 491(1–2):49
Lattig J, Bohl M, Fischer P, Tischer S, Tietbohl C, Menschikowski M, Gutzeit HO, Metz P, Pisabarro MT (2007) J Comput Aided Mol Des 21(8):473
Dahan A, Ben-Shabat S, Cohen N, Keinan S, Kurnikov I, Aponick A, Zimmermann EM (2016) Curr Top Med Chem 16(23):2543
Dahan A, Duvdevani R, Dvir E, Elmann A, Hoffman A (2007) J Control Release 119(1):86
Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E (2015) SoftwareX 1–2:19
Kumar S, Rosenberg JM, Bouzida D, Swendsen RH, Kollman PA (1992) J Comput Chem 13(8):1011
Straatsma TP, Berendsen HJC (1988) J Chem Phys 89(9):5876
Chodera JD, Mobley DL, Shirts MR, Dixon RW, Branson K, Pande VS (2011) Curr Opin Struct Biol 21(2):150
Dvir E, Friedman JE, Lee JY, Koh JY, Younis F, Raz S, Shapiro I, Hoffman A, Dahan A, Rosenberg G, Angel I, Kozak A, Duvdevani R (2006) J Pharmacol Exp Ther 318(3):1248
Dahan A, Hoffman A (2007) Drug Metab Dispos 35(2):321
Dvir E, Elman A, Simmons D, Shapiro I, Duvdevani R, Dahan A, Hoffman A, Friedman JE (2007) CNS Drug Rev 13(2):260
Dahan A, Duvdevani R, Shapiro I, Elmann A, Finkelstein E, Hoffman A (2008) J Control Release 126(1):1
Acknowledgements
This work was funded through the US-Israel Binational Science Foundation (BSF) Grant No: 2015365. This work is a part of Ms. Milica Markovic PhD dissertation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Dahan, A., Markovic, M., Keinan, S. et al. Computational modeling and in-vitro/in-silico correlation of phospholipid-based prodrugs for targeted drug delivery in inflammatory bowel disease. J Comput Aided Mol Des 31, 1021–1028 (2017). https://doi.org/10.1007/s10822-017-0079-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-017-0079-5