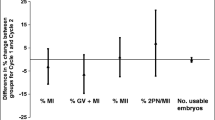
Abstract
Purpose
In vitro maturation (IVM) is a technology that generates mature oocytes following culture of immature cumulus-oocyte complexes (COC) in vitro. IVM is characterized by minimal patient stimulation, making it attractive for certain patient groups. Recently, a biphasic IVM system, capacitation (CAPA)-IVM, has shown improved clinical outcomes relative to standard IVM; however, it remains less efficient than IVF. This study assessed whether supplementation of CAPA-IVM culture media with the novel TGFβ superfamily proteins cumulin and super-GDF9 improves subsequent mouse embryo development.
Methods
Immature mouse COCs were cultured by standard IVM or biphasic IVM ± cumulin or super-GDF9.
Results
Both cumulin and super-GDF9 in standard IVM significantly improved day-6 blastocyst rate (53.9% control, 73.6% cumulin, 70.4% super-GDF9; p = 0.006; n = 382–406 oocytes). Cumulin or super-GDF9 in CAPA-IVM did not alter embryo yield or blastocyst cell allocation in an unstimulated model. Moreover, cumulin did not alter these outcomes in a mild PMSG stimulation model. Cumulin in CAPA-IVM significantly increased cumulus cell expression of cumulus expansion genes (Ptgs2, Ptx3, Adamts1, Gfat2) and decreased Lhr expression relative to control. However, cumulin-induced mRNA expression of cumulus cell (Ptgs2, Ptx3) and oocyte genes (Gdf9, Bmp15, Oct4, Stella) in CAPA-IVM remained significantly lower than that of in vivo matured cells.
Conclusion
Cumulin did not provide an additional beneficial effect in biphasic IVM in terms of blastocyst yield and cell allocation; however in standard IVM, cumulin and super-GDF9 significantly improve oocyte developmental competence.







Similar content being viewed by others
References
De Vos M, et al. The definition of IVM is clear-variations need defining. Hum Reprod. 2016;31(11):2411–5.
Vuong LN, et al. The place of in vitro maturation in assisted reproductive technology. Fertil Reproduction. 2019;01(01):11–5.
Rose BI, Laky D, Miller B. The case for in vitro maturation lower cost and more patient friendly. J Reprod Med. 2014;59(11–12):571–8.
Braam SC, et al. In-vitro maturation versus IVF: a cost-effectiveness analysis. Reprod Biomed Online. 2021;42(1):143–9.
Walls ML, et al. In vitro maturation as an alternative to standard in vitro fertilization for patients diagnosed with polycystic ovaries: a comparative analysis of fresh, frozen and cumulative cycle outcomes. Hum Reprod. 2015;30(1):88–96.
Ho VNA, et al. The effectiveness and safety of in vitro maturation of oocytes versus in vitro fertilization in women with a high antral follicle count. Hum Reprod. 2019;34(6):1055–64.
Gilchrist RB. Recent insights into oocyte-follicle cell interactions provide opportunities for the development of new approaches to in vitro maturation. Reprod Fertil Dev. 2011;23(1):23–31.
Richani D, et al. Metabolic co-dependence of the oocyte and cumulus cells: essential role in determining oocyte developmental competence. Hum Reprod Update. 2021;27(1):27–47.
Zhao Y, et al. Capacitation IVM improves cumulus function and oocyte quality in minimally stimulated mice. J Assist Reprod Genet. 2020;37(1):77–88.
Romero, S., et al., Immature oocytes from unprimed juvenile mice become a valuable source for embryo production when using c-type natriuretic peptide as essential component of culture medium. Biol Reprod, 2016. 95(3): p. 64, 1–10.
Sanchez F, et al. An improved IVM method for cumulus-oocyte complexes from small follicles in polycystic ovary syndrome patients enhances oocyte competence and embryo yield. Hum Reprod. 2017;32(10):2056–68.
Franciosi F, et al. Natriuretic peptide precursor C delays meiotic resumption and sustains gap junction-mediated communication in bovine cumulus-enclosed oocytes. Biol Reprod. 2014;91(3):61.
Santiquet NW, et al. A pre-in vitro maturation medium containing cumulus oocyte complex ligand-receptor signaling molecules maintains meiotic arrest, supports the cumulus oocyte complex and improves oocyte developmental competence. Mol Hum Reprod. 2017;23(9):594–606.
Zhang T, et al. Effect of C-type natriuretic peptide pretreatment on in vitro bovine oocyte maturation. In Vitro Cell Dev Biol Anim. 2017;53(3):199–206.
Sanchez F, et al. Biphasic in vitro maturation (CAPA-IVM) specifically improves the developmental capacity of oocytes from small antral follicles. J Assist Reprod Genet. 2019;36(10):2135–44.
Zhang J, et al. Effect of c-type natriuretic peptide on maturation and developmental competence of goat oocytes matured in vitro. PLoS One. 2015;10(7):e0132318.
Vuong LN, et al. Live births after oocyte in vitro maturation with a prematuration step in women with polycystic ovary syndrome. J Assist Reprod Genet. 2020;37(2):347–57.
Vuong LN, et al. In-vitro maturation of oocytes versus conventional IVF in women with infertility and a high antral follicle count: a randomized non-inferiority controlled trial. Hum Reprod. 2020;35(11):2537–47.
Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14(2):159–77.
Dong J, et al. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383(6600):531–5.
Galloway SM, et al. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet. 2000;25(3):279–83.
Hussein TS, Thompson JG, Gilchrist RB. Oocyte-secreted factors enhance oocyte developmental competence. Dev Biol. 2006;296(2):514–21.
Gilchrist RB, et al. Molecular basis of oocyte-paracrine signalling that promotes granulosa cell proliferation. J Cell Sci. 2006;119(Pt 18):3811–21.
Sugimura S, et al. Amphiregulin co-operates with bone morphogenetic protein 15 to increase bovine oocyte developmental competence: effects on gap junction-mediated metabolite supply. Mol Hum Reprod. 2014;20(6):499–513.
Mottershead DG, Ritter LJ, Gilchrist RB. Signalling pathways mediating specific synergistic interactions between GDF9 and BMP15. Mol Hum Reprod. 2012;18(3):121–8.
Mottershead DG, et al. Cumulin, an oocyte-secreted heterodimer of the transforming growth factor-beta family, is a potent activator of granulosa cells and improves oocyte quality. J Biol Chem. 2015;290(39):24007–20.
Stocker WA, et al. A variant of human growth differentiation factor-9 that improves oocyte developmental competence. J Biol Chem. 2020;295(23):7981–91.
Simpson CM, et al. Activation of latent human GDF9 by a single residue change (Gly 391 Arg) in the mature domain. Endocrinology. 2012;153(3):1301–10.
Thouas GA, et al. Simplified technique for differential staining of inner cell mass and trophectoderm cells of mouse and bovine blastocysts. Reprod Biomed Online. 2001;3(1):25–9.
Cakmak H, et al. Dynamic secretion during meiotic reentry integrates the function of the oocyte and cumulus cells. Proc Natl Acad Sci U S A. 2016;113(9):2424–9.
Akin N, et al. Glucose metabolism characterization during mouse in vitro maturation identifies alterations in cumulus cellsdagger. Biol Reprod. 2021;104(4):902–13.
Gilchrist RB, et al. Oocyte maturation and quality: role of cyclic nucleotides. Reproduction. 2016;152(5):R143–57.
Funahashi H, Cantley TC, Day BN. Synchronization of meiosis in porcine oocytes by exposure to dibutyryl cyclic adenosine monophosphate improves developmental competence following in vitro fertilization. Biol Reprod. 1997;57(1):49–53.
Luciano AM, et al. Effect of different levels of intracellular cAMP on the in vitro maturation of cattle oocytes and their subsequent development following in vitro fertilization. Mol Reprod Dev. 1999;54(1):86–91.
Thomas RE, et al. Effect of specific phosphodiesterase isoenzyme inhibitors during in vitro maturation of bovine oocytes on meiotic and developmental capacity. Biol Reprod. 2004;71(4):1142–9.
Albuz FK, et al. Simulated physiological oocyte maturation (SPOM): a novel in vitro maturation system that substantially improves embryo yield and pregnancy outcomes. Hum Reprod. 2010;25(12):2999–3011.
Zhang M, et al. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330(6002):366–9.
Norris RP, et al. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development. 2009;136(11):1869–78.
Vaccari S, et al. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod. 2009;81(3):595–604.
Kirillova A, et al. Improved maturation competence of ovarian tissue oocytes using a biphasic in vitro maturation system for patients with gynecological malignancy: a study on sibling oocytes. J Assist Reprod Genet. 2021;38(6):1331–40.
Peng J, et al. Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci U S A. 2013;110(8):E776–85.
McIntosh CJ, et al. The proregion of mouse BMP15 regulates the cooperative interactions of BMP15 and GDF9. Biol Reprod. 2008;79(5):889–96.
Heath DA, Pitman JL, McNatty KP. Molecular forms of ruminant BMP15 and GDF9 and putative interactions with receptors. Reproduction. 2017;154(4):521–34.
Mottershead DG, et al. Growth differentiation factor 9:bone morphogenetic protein 15 (GDF9:BMP15) synergism and protein heterodimerization. Proc Natl Acad Sci U S A. 2013;110(25):E2257.
De Vos M, et al. Perspectives on the development and future of oocyte IVM in clinical practice. J Assist Reprod Genet. 2021;38(6):1265–80.
Eppig JJ, Schroeder AC, O’Brien MJ. Developmental capacity of mouse oocytes matured in vitro: effects of gonadotrophic stimulation, follicular origin and oocyte size. J Reprod Fertil. 1992;95(1):119–27.
Gilchrist RB, Nayudu PL, Hodges JK. Maturation, fertilization, and development of marmoset monkey oocytes in vitro. Biol Reprod. 1997;56(1):238–46.
El-Hayek S, Demeestere I, Clarke HJ. Follicle-stimulating hormone regulates expression and activity of epidermal growth factor receptor in the murine ovarian follicle. Proc Natl Acad Sci U S A. 2014;111(47):16778–83.
Franciosi F, Manandhar S, Conti M. FSH regulates mRNA translation in mouse oocytes and promotes developmental competence. Endocrinology. 2016;157(2):872–82.
Mikkelsen AL, Smith SD, Lindenberg S. In-vitro maturation of human oocytes from regularly menstruating women may be successful without follicle stimulating hormone priming. Hum Reprod. 1999;14(7):1847–51.
Fadini R, et al. Effect of different gonadotrophin priming on IVM of oocytes from women with normal ovaries: a prospective randomized study. Reprod Biomed Online. 2009;19(3):343–51.
Luciano AM, Sirard MA. Successful in vitro maturation of oocytes: a matter of follicular differentiation. Biol Reprod. 2018;98(2):162–9.
Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice. Dev Biol. 2007;305(1):300–11.
Su YQ, et al. Mouse oocytes enable LH-induced maturation of the cumulus-oocyte complex via promoting EGF receptor-dependent signaling. Mol Endocrinol. 2010;24(6):1230–9.
Ritter LJ, Sugimura S, Gilchrist RB. Oocyte induction of EGF responsiveness in somatic cells is associated with the acquisition of porcine oocyte developmental competence. Endocrinology. 2015;156(6):2299–312.
Sugimura S, et al. Promotion of EGF receptor signaling improves the quality of low developmental competence oocytes. Dev Biol. 2015;403(2):139–49.
Richani D, Gilchrist RB. The epidermal growth factor network: role in oocyte growth, maturation and developmental competence. Hum Reprod Update. 2018;24(1):1–14.
Li HJ, et al. Extending prematuration with cAMP modulators enhances the cumulus contribution to oocyte antioxidant defence and oocyte quality via gap junctions. Hum Reprod. 2016;31(4):810–21.
Richani D, et al. Pre-maturation with cAMP modulators in conjunction with EGF-like peptides during in vitro maturation enhances mouse oocyte developmental competence. Mol Reprod Dev. 2014;81(5):422–35.
Richani D, Gilchrist RB (2021) Approaches to oocyte meiotic arrest in vitro and impact on oocyte developmental competence. Biol Reprod.
Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update. 2007;13(3):289–312.
Li R, et al. Oocyte-secreted factor(s) determine functional differences between bovine mural granulosa cells and cumulus cells. Biol Reprod. 2000;63(3):839–45.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nazli Akin and Dulama Richani are joint first authors.
Ellen Anckaert and Robert B. Gilchrist are joint last authors.
Rights and permissions
About this article
Cite this article
Akin, N., Richani, D., Liao, X. et al. Effect of cumulin and super-GDF9 in standard and biphasic mouse IVM. J Assist Reprod Genet 39, 127–140 (2022). https://doi.org/10.1007/s10815-021-02382-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02382-z