Abstract
Purpose
The aim was to describe the first experience with fertility preservation by cryopreservation of ovarian tissue (OTC) in pre-pubertal girls with galactosemia and further to characterize ovarian follicular morphology and expression of proteins important for ovarian function.
Methods
Retrospectively, follicle density was estimated in ovarian cortical tissues from 6 pre-pubertal girls below the age of 12 years diagnosed with galactosemia and from 31 girls below the age of 18 years who had one ovary removed for fertility preservation for other reasons prior to gonadotoxic treatment. Additionally, expression of 4 glycoproteins important for follicle development were analyzed with immunohistochemistry in two galactosemic ovaries (aged 0.9 and 1.7 years) and compared to normal age-matched controls. The proteins included were: anti-Müllerian hormone (AMH) pro-mature and C-terminal, growth differentiation factor-9 (GDF-9), bone morphogenetic protein 15 (BMP-15), and pregnancy-associated plasma protein A (PAPP-A).
Results
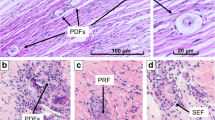
Girls with galactosemia below the age of 5 years presented with morphological normal follicles and follicle densities within the 95% confidence interval (CI) of controls. No follicles were detected in the ovary from an 11.7-year-old girl with galactosemia. Expression of AMH, GDF-9, BMP-15, and PAPP-A appeared similar in follicles from girls with galactosemia and controls.
Conclusions
These findings suggest that young girls with galactosemia maintain follicles in early childhood and fertility cryopreservation may be considered an option in this patient group. The pathophysiology of galactosemia leading to an accelerated follicle loss is unknown and it is currently unknown to what extent transplanted ovarian tissue can sustain fertility in adult life.



Similar content being viewed by others
References
Suzuki M, West C, Beutler E. Large-scale molecular screening for galactosemia alleles in a pan-ethnic population. Hum Genet. 2001;109:210–5.
Gubbels CS, Land JA, Rubio-Gozalbo ME. Fertility and impact of pregnancies on the mother and child in classic galactosemia. Obstet Gynecol Surv. 2008;63:334–43.
Rubio-Gozalbo ME, Gubbels CS, Bakker JA, Menheere PP, Wodzig WK, Land JA. Gonadal function in male and female patients with classic galactosemia. Hum Reprod Update. 2010;16:177–88.
Sanders RD, Spencer JB, Epstein MP, Pollak SV, Vardhana PA, Lustbader JW, et al. Biomarkers of ovarian function in girls and women with classic galactosemia. Fertil Steril. 2009;92:344–51.
Spencer JB, Badik JR, Ryan EL, Gleason TJ, Broadaway KA, Epstein MP, et al. Modifiers of ovarian function in girls and women with classic galactosemia. J Clin Endocrinol Metab. 2013;98:E1257–65.
Waggoner DD, Buist NRM, Donnell GN. Long-term prognosis in galactosaemia: results of a survey of 350 cases. J Inherit Metab Dis. 1990;13:802–18.
Guerrero NV, Singh RH, Manatunga A, Berry GT, Steiner RD, Elsas LJ. Risk factors for premature ovarian failure in females with galactosemia. J Pediatr. 2000;137:833–41.
Fridovich-Keil JL, Gubbels CS, Spencer JB, Sanders RD, Land JA, Rubio-Gozalbo E. Ovarian function in girls and women with GALT-deficiency galactosemia. J Inherit Metab Dis. 2011;34:357–66.
Varela-Lema L, Paz-Valinas L, Atienza-Merino G, Zubizarreta-Alberdi R, Villares RV, López-García M. Appropriateness of newborn screening for classic galactosaemia: a systematic review. J Inherit Metab Dis. 2016;39:633–49.
van Erven B, Berry GT, Cassiman D, Connolly G, Forga M, Gautschi M, et al. Fertility in adult women with classic galactosemia and primary ovarian insufficiency. Fertil Steril. 2017;108:168–74.
Rebar RW. Premature ovarian failure. Obstet Gynecol. 2009;113:1355–63.
van Kasteren YM, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update. 1999;5:483–92.
Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360:606–14.
Thakur M, Feldman G, Puscheck EE. Primary ovarian insufficiency in classic galactosemia: current understanding and future research opportunities. J Assist Reprod Genet. 2018;35:3–16.
Gubbels CS, Land JA, Evers JLH, Bierau J, Menheere PPCA, Robben SGF, et al. Primary ovarian insufficiency in classic galactosemia: role of FSH dysfunction and timing of the lesion. J Inherit Metab Dis. 2013;36:29–34.
Forges T, Monnier-Barbarino P, Leheup B, Jouvet P. Pathophysiology of impaired ovarian function in galactosaemia. Hum Reprod Update. 2006;12:573–84.
Xu YK, Ng WG, Kaufman FR, Lobo RA, Donnell GN. Galactose metabolism in human ovarian tissue. Pediatr Res. 1989;25:151–5.
Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P, Andersen CY. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet. 2017;34:325–36.
Gellert SE, Pors SE, Kristensen SG, Bay-Bjørn AM, Ernst E, Yding Andersen C. Transplantation of frozen-thawed ovarian tissue: an update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J Assist Reprod Genet. 2018;Epub ahead.
van Erven B, Gubbels CS, van Golde RJ, Dunselman GA, Derhaag JG, de Wert G, et al. Fertility preservation in female classic galactosemia patients. Orphanet J Rare Dis. 2013;8:107.
Issaoui ME, Giorgione V, Mamsen LS, Rechnitzer C, Birkebaek N, Clausen N, et al. Effect of first line cancer treatment on the ovarian reserve and follicular density in girls under the age of 18 years. Fertil Steril. 2016;6:1757–62.
Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23:2266–72.
Rosendahl M, Andersen CY, Ernst E, Westergaard LG, Rasmussen PE, Loft A, et al. Ovarian function after removal of an entire ovary for cryopreservation of pieces of cortex prior to gonadotoxic treatment: a follow-up study. Hum Reprod. 2008;23:2475–83.
Rosendahl M, Timmermans Wielenga V, Nedergaard L, Kristensen SG, Ernst E, Rasmussen PE, et al. Cryopreservation of ovarian tissue for fertility preservation: no evidence of malignant cell contamination in ovarian tissue from patients with breast cancer. Fertil Steril. 2011;95:2158–61.
Schmidt KLT, Byskov AG, Andersen AN, Müller J, Andersen CY. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod. 2003;18:1158–64.
Westergaard CG, Byskov AG, Andersen CY. Morphometric characteristics of the primordial to primary follicle transition in the human ovary in relation to age. Hum Reprod. 2007;22:2225–31.
McLaughlin M, Kelsey TW, Wallace WHB, Anderson RA, Telfer EE. An externally validated age-related model of mean follicle density in the cortex of the human ovary. J Assist Reprod Genet. 2015;32:1089–95.
Andersen CY, Schmidt KT, Kristensen SG, Rosendahl M, Byskov AG, Ernst E. Concentrations of AMH and inhibin-B in relation to follicular diameter in normal human small antral follicles. Hum Reprod. 2010;25:1282–7.
Jeppesen J, Anderson RA, Kelsey TW, Christiansen SL, Kristensen SG, Jayaprakasan K, et al. Which follicles make the most anti-Müllerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol Hum Reprod. 2013;19:519–27.
Robertson DM, Kumar A, Kalra B, Shah S, Pruysers E, Vanden BH, et al. Detection of serum antimullerian hormone in women approaching menopause using sensitive antimullerian hormone enzyme-linked immunosorbent assays. Menopause. 2014;21:1–10.
McGrath SA, Esquela AF, Lee SJ. Oocyte-specific expression of growth/differentiation factor-9. Mol Endocrinol. 1995;9:131–6.
Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–5.
Bøtkjær JA, Jeppesen JV, Wissing ML, Kløverpris S, Oxvig C, Mason JI, et al. Pregnancy-associated plasma protein A in human ovarian follicles and its association with intrafollicular hormone levels. Fertil Steril. 2015;104:1294–301.
Weenen C, Laven JSE, von Bergh ARM, Cranfield M, Groome NP, Visser JA, et al. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83.
Sidis Y, Fujiwara T, Leykin L, Isaacson K, Toth T, Schneyer AL. Characterization of inhibin/activin subunit, activin receptor, and follistatin messenger ribonucleic acid in human and mouse oocytes: evidence for activin’s paracrine signaling from granulosa cells to oocytes. Biol Reprod. 1998;59:807–12.
Liu G, Shi F, Blas-Machado U, Yu R, Davis VL, Foster WG, et al. Dietary galactose inhibits GDF-9 mediated follicular development in the rat ovary. Reprod Toxicol. 2006;21:26–33.
Aaltonen J, Laitinen MP, Vuojolainen K, Jaatinen R, Horelli-Kuitunen N, Seppä L, et al. Human growth differentiation factor 9 (GDF-9) and its novel homolog GDF-9B are expressed in oocytes during early folliculogenesis. J Clin Endocrinol Metab. 1999;84:2744–50.
Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science. 2004;305(80):1157–9.
Wang J, Roy SK. Growth differentiation factor-9 and stem cell factor promote primordial follicle formation in the hamster: modulation by follicle-stimulating hormone. Biol Reprod. 2004;70:577–85.
Kedem A, Fisch B, Garor R, Ben-Zaken A, Gizunterman T, Felz C, et al. Growth differentiating factor 9 (GDF9) and bone morphogenetic protein 15 both activate development of human primordial follicles in vitro, with seemingly more beneficial effects of GDF9. J Clin Endocrinol Metab. 2011;96:E1246–54.
Bayne RAL, Kinnell HL, Coutts SM, He J, Childs AJ, Anderson RA. GDF9 is transiently expressed in oocytes before follicle formation in the human fetal ovary and is regulated by a novel NOBOX transcript. PLoS One Libr Sci. 2015;10:e0119819.
Kristensen SG, Andersen K, Clement CA, Franks S, Hardy K, Andersen CY. Expression of TGF-beta superfamily growth factors, their receptors, the associated SMADs and antagonists in five isolated size-matched populations of pre-antral follicles from normal human ovaries. Mol Hum Reprod. 2014;20:293–308.
Wei L-N, Huang R, Li L-L, Fang C, Li Y, Liang X-Y. Reduced and delayed expression of GDF9 and BMP15 in ovarian tissues from women with polycystic ovary syndrome. J Assist Reprod Genet. 2014;31:1483–90.
Jepsen MR, Kløverpris S, Bøtkjær JA, Wissing ML, Andersen CY, Oxvig C. The proteolytic activity of pregnancy-associated plasma protein-A is potentially regulated by stanniocalcin-1 and -2 during human ovarian follicle development. Hum Reprod. 2016;31:866–74.
Levy H, Driscoll S, Porensky R, Wender D. Ovarian failure in galactosemia. N Engl J Med. 1984;310:50.
Beauvais P, Guilhaume A. Ovarian insufficiency in congenital galactosemia. Presse Med. 1984;13:2685–7.
Morrow RJ, Atkinson AB, Carson DJ, Sloan JM, Traub AI. Ovarian failure in a young woman with galactosaemia. Ulster Med J. 1985;54:218–20.
Kaufman FR, Xu YK, Ng WG, Silva PD, Lobo RA, Donnell GN. Gonadal function and ovarian galactose metabolism in classic galactosemia. Acta Endocrinol. 1989;120:129–33.
Poirot C, Abirached F, Prades M, Coussieu C, Bernaudin F, Piver P. Induction of puberty by autograft of cryopreserved ovarian tissue. Lancet. 2012;379:588.
Ernst E, Kjærsgaard M, Birkebæk NH, Clausen N, Andersen CY. Case report: stimulation of puberty in a girl with chemo- and radiation therapy induced ovarian failure by transplantation of a small part of her frozen/thawed ovarian tissue. Eur J Cancer. 2013;49(4):911–4.
Beckmann MW, Dittrich R, Lotz L, van der Ven K, van der Ven HH, Liebenthron J, et al. Fertility protection: complications of surgery and results of removal and transplantation of ovarian tissue. Reprod BioMed Online. 2018;36:188–96.
Jensen AK, Kristensen SG, MacKlon KT, Jeppesen JV, Fedder J, Ernst E, et al. Outcomes of transplantations of cryopreserved ovarian tissue to 41 women in Denmark. Hum Reprod. 2015;30:2838–45.
Acknowledgements
We thank Marianne Sguazzino for the excellent technical assistance.
Authors’ role
L.S.M. and C.Y.A. designed and drafted the study. L.S.M. was responsible for the IHC staining and estimating the follicle densities in ovaries from patients with galactosemia. T.W.K. designed the mathematics for follicle density estimation and developed the density prediction model. E.E. and K.T.M. did the initial fertility preservation counseling with the parents and was responsible for the collection of the tissue. A.M.L. is the physician in charge of the girls with galactosemia and obtained their genetic information. The final manuscript was approved by all authors.
Funding
The Novo Nordisk Foundation and the EU interregional project ReproUnion funded this study. They had no role in the study design, collection and analysis of data, and data interpretation or in writing the report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Scientific Ethical Committee for the Capital Region (KF (01) 170/99).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mamsen, L.S., Kelsey, T.W., Ernst, E. et al. Cryopreservation of ovarian tissue may be considered in young girls with galactosemia. J Assist Reprod Genet 35, 1209–1217 (2018). https://doi.org/10.1007/s10815-018-1209-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-018-1209-2