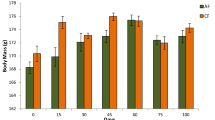
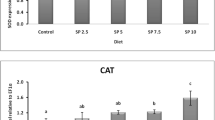
Abstract
Microalgae are major antioxidant producers and feed containing these substances is known to be beneficial. Microalgae cultivation is an alternative way to produce biodiesel and, after oil extraction, residual algal biomass (RAB) is obtained. The RAB was tested as an ingredient in fish feed production and its safety evaluation is important to prevent risks to fish health. This study aim was to evaluate, through biochemical and genetic biomarkers, the safety of RAB in catfish, Rhamdia quelen, feed. Acutodesmus obliquus microalgae RAB, cultivated in Chu medium, was used in feed formulation. A standard feed without RAB (0%) was produced, and three other feeds were enriched with RAB in 1, 2, and 3% proportion. Each feed kind was given to a 15 R. quelen fingerling group for 60 days. The evaluated biochemical biomarkers were superoxide dismutase (SOD) and catalase (CAT) activities, lipid peroxidation (LPO) in the liver, and acetilcolinesterase (AChE) activity in the brain and muscles. The genetic biomarkers analyzed were halo assay in erythrocytes, comet assay in erythrocytes, liver and brain, and piscine micronucleus. The SOD activity was increased in the 3% group; CAT activity and LPO levels were not different among the groups. In the comet assay, a significant decrease in DNA damage in erythrocytes (2 and 3%) and liver tissue (3%) was observed. In the brain, DNA damage was not observed. These results corroborate that as the RAB amount increased, the organisms showed a potential antioxidant effect, as the 3% RAB feed had the best results.
Similar content being viewed by others
References
Abdel-Daim M, El-Bialy BE, Rahman HGA, Radi AM, Hefny HA, Hassan AM (2016) Antagonistic effects of Spirulina platensis against sub-acute deltamethrin toxicity in mice: biochemical and histopathological studies. Biomed Pharmacother 77:79–85
Abdelkhalek NKM, Ghazy EW, Abdel-Daim MM (2015) Pharmacodynamic interaction of Spirulina platensis and deltamethrin in freshwater fish Nile tilapia, Oreochromis niloticus: impact on lipid peroxidation and oxidative stress. Environ Sci Pollut Res 22:3023–3031
Abdelkhalek NKM, Eissa IAM, Ahmed E, Kilany OE, El-Adl M, Dawood MAO, Hassan AM, Abdel-Daim MM (2017) Protective role of dietary Spirulina platensis against diazinon-induced oxidative damage in Nile tilapia; Oreochromis niloticus. Environ Toxicol Pharmacol 54:99–104
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Andrade VM, de Freitas TRO, da Silva J (2004) Comet assay using mullet (Mugil sp.) and sea catfish (Netuma sp.) erythrocytes for the detection of genotoxic pollutants in aquatic environment. Mutat Res 560:57–67
Azqueta A, Collins AR (2012) Carotenoids and DNA damage. Mutation Research, Fundamental and Molecular Mechanisms of Mutagenesis 733:4–13
Azqueta A, Shaposhnikov S, Collins AR (2009) DNA oxidation: investigating its key role in environmental mutagenesis with the comet assay. Mutation Research, Genetic Toxicology and Environmental Mutagenesis 674:101–108
Balen RE, Geraldo E, Marques AEML, Cestari MM, Vargas JVC, Corrêa DDO, Bellin MA, Meurer F (2015) Effect of defatted microalgae (Scenedesmus obliquus) biomass inclusion on growth performance of Rhamdia quelen (Quoy & Gaimard, 1824). J Appl Ichthyol 31:98–101
Barreto A, Luis LG, Soares AMVM, Paíga P, Santos LHMLM, Delerue-Matos C, Hylland K, Loureiro S, Oliveira M (2017) Genotoxicity of gemfibrozil in the gilthead seabream (Sparus aurata). Mutation Research, Genetic Toxicology and Environmental Mutagenesis 821:36–42
Becker AG, Parodi TV, Gonçalves JF, Bagatini MD, Spanevello RM, Morsch VM, Chitolina Schetinger MR, Baldisserotto B (2013) Ectonucleotidase and acetylcholinesterase activities in silver catfish (Rhamdia quelen) exposed to different salinities. Biochem Syst Ecol 46:44–49
Bishop NI, Urbig T, Senger H (1995) Complete separation of the β,ε- and β,β-carotenoid biosynthetic pathways by a unique mutation of the lycopene cyclase in the green alga, Scenedesmus obliquus. FEBS Lett 367:158–162
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bretaud S, Toutant J-P, Saglio P (2000) Effects of carbofuran, diuron, and nicosulfuron on acetylcholinesterase activity in goldfish (Carassius auratus). Ecotoxicol Environ Saf 47:117–124
Canton R, Weingartner M, Fracalossi DM, Zaniboni Filho E (2007) Influência da freqüência alimentar no desempenho de juvenis de jundiá. Rev Bras Zootec 36:749–753
Carrasco KR, Tilbury KL, Myers MS (1990) Assessment of the piscine micronucleus test as an in situ biological indicator of chemical contaminant effects. Can J Fish Aquat Sci 47:2123–2136
Cattaneo R, Clasen B, Loro VL, De Menezes CC, Pretto A, Baldisserotto B, Santi A, De Avila LA (2011) Toxicological responses of Cyprinus carpio exposed to a commercial formulation containing glyphosate. Bull Environ Contam Toxicol 87:597–602
Cemeli E, Baumgartner A, Anderson D (2009) Antioxidants and the comet assay. Mutat Res 681:51–67
Cestari MM, Lemos PMM, Ribeiro CADO, Costa JRMA, Pelletier E, Ferraro MVM, Mantovani MS, Fenocchio AS (2004) Genetic damage induced by trophic doses of lead in the neotropical fish Hoplias malabaricus (Characiformes, Erythrinidae) as revealed by the comet assay and chromosomal aberrations. Genet Mol Biol 27:270–274
Chu SP (1942) The influence of the mineral composition of the medium on the growth of planktonic algae: part I. methods and culture media. J Ecol 30:284–325
Collins AR (2001) Carotenoids and genomic stability. Mutation Research, Fundamental and Molecular Mechanisms of Mutagenesis 475:21–28
Collins AR, Ai-guo M, Duthie SJ (1995) The kinetics of repair of oxidative DNA damage (strand breaks and oxidised pyrimidines) in human cells. Mutation Research, DNA Repair 336:69–77
Damergi E, Schwitzguébel JP, Refardt D, Sharma S, Holliger C, Ludwig C (2017) Extraction of carotenoids from Chlorella vulgaris using green solvents and syngas production from residual biomass. Algal Res 25:488–495
Del Campo JA, García-González M, Guerrero MG (2007) Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Appl Microbiol Biotechnol 74:1163–1174
Ellman GL, Courtney KD, Andres V, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Escorsim AM, da Rocha G, Vargas JVC, Mariano AB, Ramos LP, Corazza ML, Cordeiro CS (2018) Extraction of Acutodesmus obliquus lipids using a mixture of ethanol and hexane as solvent. Biomass Bioenergy 108:470–478
Ferraro MVM, Fenocchio AS, Mantovani MS, Ribeiro CADO, Cestari MM (2004) Mutagenic effects of tributyltin and inorganic lead (Pb II) on the fish H. malabaricus as evaluated using the comet assay and the piscine micronucleus and chromosome aberration tests. Genet Mol Biol 27:103–107
Frenzilli G, Nigro M, Lyons BP (2009) The comet assay for the evaluation of genotoxic impact in aquatic environments. Mutat Res 681:80–92
Gao R, Yuan Z, Zhao Z, Gao X (1998) Mechanism of pyrogallol autoxidation and determination of superoxide dismutase enzyme activity. Bioelectrochem Bioenerg 45:41–45
Ghelfi A, Ribas JLC, Guiloski IC, Bettim FL, Piancini LDS, Cestari MM, Pereira AJ, Sassaki GL, Silva de Assis HC (2016) Evaluation of biochemical, genetic and hematological biomarkers in a commercial catfish Rhamdia quelen exposed to diclofenac. Bull Environ Contam Toxicol 96:49–54
Ghisi NDC, Ramsdorf WA, Ferraro MVM, Almeida MIMD, Ribeiro CADO, Cestari MM (2011) Evaluation of genotoxicity in Rhamdia quelen (Pisces, Siluriformes) after sub-chronic contamination with Fipronil. Environ Monit Assess 180:589–599
Gomes L d C, Golombieski JI, Gomes ARC, Baldisserotto B (2000) Biologia do jundiá Rhamdia quelen (Teleostei, Pimelodidae). Ciência Rural 30:179–185
Gontijo ÁM d MC, Barreto RE, Speit G, Valenzuela Reyes VA, Volpato GL, Favero Salvadori DM (2003) Anesthesia of fish with benzocaine does not interfere with comet assay results. Mutation Research, Genetic Toxicology and Environmental Mutagenesis 534:165–172
Guedes AC, Amaro HM, Pereira RD, Malcata FX (2011) Effects of temperature and pH on growth and antioxidant content of the microalga Scenedesmus obliquus. Biotechnol Prog 27:1218–1224
Guiloski IC, Stein Piancini LD, Dagostim AC, de Morais Calado SL, Fávaro LF, Boschen SL, Cestari MM, da Cunha C, Silva de Assis HC (2017) Effects of environmentally relevant concentrations of the anti-inflammatory drug diclofenac in freshwater fish Rhamdia quelen. Ecotoxicol Environ Saf 139:291–300
He Q, Yang H, Wu L, Hu C (2015) Effect of light intensity on physiological changes, carbon allocation and neutral lipid accumulation in oleaginous microalgae. Bioresour Technol 191:219–228
Heddle JA (1973) A rapid in vivo test for chromosomal damage. Mutat Res 18:187–190
Jiang Z-Y, Hunt JV, Wolff SP (1992) Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem 202:384–389
Kaizer RR, Loro VL, Schetinger MRC, Morsch VM, Tabaldi LA, da Rosa CS, Garcia LDO, Becker AG, Baldisserotto B (2009) NTPDase and acetylcholinesterase activities in silver catfish, Rhamdia quelen (Quoy & Gaimard, 1824) (Heptapteridae) exposed to interaction of oxygen and ammonia levels. Neotropical Ichthyology 7:635–640
Kochhann D, Pavanato MA, Llesuy SF, Correa LM, Konzen Riffel AP, Loro VL, Mesko MF, Flores ÉMM, Dressler VL, Baldisserotto B (2009) Bioaccumulation and oxidative stress parameters in silver catfish (Rhamdia quelen) exposed to different thorium concentrations. Chemosphere 77:384–391
Krinsky NI, Johnson EJ (2005) Carotenoid actions and their relation to health and disease. Mol Asp Med 26:459–516
Löf M, Sundelin B, Liewenborg B, Bandh C, Broeg K, Schatz S, Gorokhova E (2016) Biomarker-enhanced assessment of reproductive disorders in Monoporeia affinis exposed to contaminated sediment in the Baltic Sea. Ecol Indic 63:187–195
Miron DS, Crestani M, Rosa Shettinger M, Maria Morsch V, Baldisserotto B, Angel Tierno M, Moraes G, Vieira VLP (2005) Effects of the herbicides clomazone, quinclorac, and metsulfuron methyl on acetylcholinesterase activity in the silver catfish (Rhamdia quelen) (Heptapteridae). Ecotoxicol Environ Saf 61:398–403
Norambuena F, Hermon K, Skrzypczyk V, Emery JA, Sharon Y, Beard A, Turchini GM (2015) Algae in fish feed: performances and fatty acid metabolism in juvenile Atlantic Salmon. PLoS One 10:e0124042
Pamplona JH, Oba ET, da Silva TA, Ramos LP, Ramsdorf WA, Cestari MM, Ribeiro CAO, Zampronio AR, Silva de Assis HC (2011) Subchronic effects of dipyrone on the fish species Rhamdia quelen. Ecotoxicol Environ Saf 74:342–349
Patias LD, Fernandes AS, Petry FC, Mercadante AZ, Jacob-Lopes E, Zepka LQ (2017) Carotenoid profile of three microalgae/cyanobacteria species with peroxyl radical scavenger capacity. Food Res Int 100:260–266
Piancini LDS, Santos GS, Tincani FH, Cestari M (2015) Piscine micronucleus test and the comet assay reveal genotoxic effects of atrazine herbicide in the neotropical fish Rhamdia quelen. Ecotoxicology and Environmental Contamination 10:55–60
Quoy JRC, Gaimard P (1824) Description des Poissons. In: Freycinet LCD de (ed) Voyage autour du Monde, Entrepris par Ordre du Roi, ... Exécuté sur les corvettes de S. M. l’Uranie et la Physicienne, pendant les années 1817, 1818, 1819 et 1820. Chez Pillet Aîné, Paris, pp 192–401. https://biodiversitylibrary.org/page/40871183
Ramsdorf WA, Guimarães F de SF, Ferraro MVM, Gabardo J, Trindade ES, Cestari MM (2009) Establishment of experimental conditions for preserving samples of fish blood for analysis with both comet assay and flow cytometry. Mutation Research, Genetic Toxicology and Environmental Mutagenesis 673:78–81
Rao A, Rao L (2007) Carotenoids and human health. Pharmacol Res 55:207–216
Reis LCR, de Oliveira VR, Hagen MEK, Jablonski A, Flôres SH, de Oliveira Rios A (2015) Carotenoids, flavonoids, chlorophylls, phenolic compounds and antioxidant activity in fresh and cooked broccoli (Brassica oleracea var. avenger) and cauliflower (Brassica oleracea var. Alphina F1). Food Science and Technology 63:177–183
Roca M, Chen K, Pérez-Gálvez A (2016) Chlorophylls. In: Carle R, Schweiggert R (eds) Handbook on natural pigments in food and beverages. Elsevier, NY pp 125–158.
Romani R, Antognelli C, Baldracchini F, De Santis A, Isani G, Giovannini E, Rosi G (2003) Increased acetylcholinesterase activities in specimens of Sparus auratus exposed to sublethal copper concentrations. Chem Biol Interact 145:321–329
Sayed AEDH, El-Sayed YS, El-Far AH (2017) Hepatoprotective efficacy of Spirulina platensis against lead-induced oxidative stress and genotoxicity in catfish; Clarias gariepinus. Ecotoxicol Environ Saf 143:344–350
Schetinger MR, Bonan CD, Morsch VM, Bohrer D, Valentim LM, Rodrigues SR (1999) Effects of aluminum sulfate on delta-aminolevulinate dehydratase from kidney, brain, and liver of adult mice. Braz J Med Biol Res 32:761–766
Schmid W (1975) The micronucleus test. Mutat Res 31:9–15
Singh NP (2000) A simple method for accurate estimation of apoptotic cells. Exp Cell Res 256:328–337
Speit G, Hartmann A (1999) The comet assay (single-cell gel test): a sensitive genotoxicity test for the detection of DNA damage and repair. Methods Mol Biol 113:203–212
Stankevičiūtė M, Butrimavičienė L, Valskienė R, Greiciūnaitė J, Baršienė J, Vosylienė MZ, Svecevičius G (2016) Analysis of nuclear abnormalities in erythrocytes of rainbow trout (Oncorhynchus mykiss) treated with cu and Zn and after 4-, 8-, and 12-day depuration (post-treatment recovery). Mutation Research, Genetic Toxicology and Environmental Mutagenesis 797:26–35
Sturm A, da Silva de Assis H, Hansen P-D (1999) Cholinesterases of marine teleost fish: enzymological characterization and potential use in the monitoring of neurotoxic contamination. Mar Environ Res 47:389–398
van der Oost R, Beyer J, Vermeulen NP (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Ventura L, Giovannini A, Savio M, Donà M, Macovei A, Buttafava A, Carbonera D, Balestrazzi A (2013) Single cell gel electrophoresis (comet) assay with plants: research on DNA repair and ecogenotoxicity testing. Chemosphere 92:1–9
Villela IV, de Oliveira IM, Silveira JC, Dias JF, Henriques JAP, da Silva J (2007) Assessment of environmental stress by the micronucleus and comet assays on Limnoperna fortunei exposed to Guaíba hydrographic region samples (Brazil) under laboratory conditions. Mutation Research, Genetic Toxicology and Environmental Mutagenesis 628:76–86
Zeppenfeld CC, Toni C, Becker AG, Miron DDS, Parodi TV, Heinzmann BM, Barcellos LJG, Koakoski G, Rosa JGSD, Loro VL, Cunha MAD, Baldisserotto B (2014) Physiological and biochemical responses of silver catfish, Rhamdia quelen, after transport in water with essential oil of Aloysia triphylla (L’Herit) Britton. Aquaculture 418–419:101–107
Zmora O, Grosse DJ, Zou N, Samocha TM (2013) Microalga for aquaculture: Practical implications. In: Richmond A, Hu Q (eds) Handbook of microalgal Culture.2nd Edn. John Wiley & Sons, Oxford, pp 628–652
Acknowledgements
The authors would like to thank MSc. Camila da Costa Senkiv and Lúcia Gil for providing language and writing support, and the assistance of the following laboratories from Federal University of Paraná (UFPR): Animal Cytogenetics and Environmental Mutagenesis Lab., Aquaculture Technology Lab., Environmental Toxicology Lab. and NPDEAS.
Funding
This work was financially supported by CNPq and CAPES under the grant [grant numbers 40001016006P1].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marques, A.E.M.L., Balen, R.E., da Silva Pereira Fernandes, L. et al. Diets containing residual microalgae biomass protect fishes against oxidative stress and DNA damage. J Appl Phycol 31, 2933–2940 (2019). https://doi.org/10.1007/s10811-019-01825-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-01825-6