Abstract
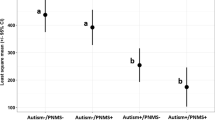
Epidemiologic studies link increased autism spectrum disorder (ASD) risk to obstetrical conditions associated with inflammation and steroid dysregulation, referred to as prenatal metabolic syndrome (PNMS). This pilot study measured steroid-related biomarkers in early second trimester maternal serum collected during the first and second trimester evaluation of risk study. ASD case and PNMS exposure status of index offspring were determined through linkage with autism registries and birth certificate records. ASD case (N = 53) and control (N = 19) groups were enriched for PNMS exposure. Higher estradiol and lower sex hormone binding globulin (SHBG) were significantly associated with increased ASD risk. Study findings provide preliminary evidence to link greater placental estradiol activity with ASD and support future investigations of the prenatal steroid environment in ASD.



Similar content being viewed by others
Change history
03 September 2019
The original version of the article has been published without funding source information.
References
Albrecht, E. D., & Pepe, G. J. (1999). Central integrative role of oestrogen in modulating the communication between the placenta and fetus that results in primate fecal-placental development. Placenta, 20(2–3), 129–139.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Philadelphia: American Psychiatric Association. https://doi.org/10.1176/appi.books.9780890425596.
Anderson, J. N., Peck, E. J., & Clark, J. H. (1975). Estrogen-induced uterine responses and growth: Relationship to receptor estrogen binding by uterine nuclei. Endocrinology, 96(1), 160–167. https://doi.org/10.1210/endo-96-1-160.
Andridge, R. R., & Little, R. J. A. (2010). A review of hot deck imputation for survey non-response. International Statistical Review = Revue Internationale de Statistique, 78(1), 40–64. https://doi.org/10.1111/j.1751-5823.2010.00103.x.
Baio, J., Wiggins, L., Christensen, D. L., Maenner, M. J., Daniels, J., & Warren, Z. (2018). Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2014. Morbidity and Mortality Weekly Report. Surveillance Summaries (Washington, D.C.: 2002), 67(6), 1–23. https://doi.org/10.15585/mmwr.ss6706a1.
Bakian, A. V., Bilder, D. A., Carbone, P. S., Hunt, T. D., Petersen, B., & Rice, C. E. (2015a). Brief report: independent validation of autism spectrum disorder case status in the Utah Autism and Developmental Disabilities Monitoring (ADDM) network site. Journal of Autism and Developmental Disorders, 45(3), 873–880. https://doi.org/10.1007/s10803-014-2187-6.
Bakian, A. V., Bilder, D. A., Coon, H., & McMahon, W. M. (2015b). Spatial relative risk patterns of autism spectrum disorders in Utah. Journal of Autism and Developmental Disorders, 45(4), 988–1000. https://doi.org/10.1007/s10803-014-2253-0.
Baron-Cohen, S., Auyeung, B., Nørgaard-Pedersen, B., Hougaard, D. M., Abdallah, M. W., Melgaard, L., et al. (2015). Elevated fetal steroidogenic activity in autism. Molecular Psychiatry, 20, 369–376.
Bilder, D. A., Bakian, A. V., Viskochil, J., Clark, E. A. S., Botts, E. L., Smith, K. R., et al. (2013). Maternal prenatal weight gain and autism spectrum disorders. Pediatrics, 132(5), e1276–e1283. https://doi.org/10.1542/peds.2013-1188.
Brown, A. S., Sourander, A., Hinkka-Yli-Salomäki, S., McKeague, I. W., Sundvall, J., & Surcel, H.-M. (2014). Elevated maternal C-reactive protein and autism in a national birth cohort. Molecular Psychiatry, 19(2), 259–264. https://doi.org/10.1038/mp.2012.197.
Center for Disease Control and Prevention, Autism and Developmental Disabilities Monitoring Network Surveillance Year 2002 Principal Investigators. (2007). Prevalence of Autism Spectrum Disorders—Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States, 2002 (MMWR No. 56) (pp. 12–28)
Corbett, B. A., Mendoza, S., Abdullah, M., Wegelin, J. A., & Levine, S. (2006). Cortisol circadian rhythms and response to stress in children with autism. Psychoneuroendocrinology, 31(1), 59–68. https://doi.org/10.1016/j.psyneuen.2005.05.011.
Coulter, C. L., & Jaffe, R. B. (1998). Functional maturation of the primate fetal adrenal in vivo: 3. Specific zonal localization and developmental regulation of CYP21A2 (P450c21) and CYP11B1/CYP11B2 (P450c11/aldosterone synthase) lead to integrated concept of zonal and temporal steroid biosynthesis. Endocrinology, 139(12), 5144–5150. https://doi.org/10.1210/endo.139.12.6333.
Cunningham, F. G. (2010). Implantation, embryogenesis, and placental development. In F. Cunningham (Ed.), Williams obstetrics, (23rd ed., pp. 36–77) New York: McGraw-Hill. Medical, c2010.
DeLong, E. R., DeLong, D. M., & Clarke-Pearson, D. L. (1988). Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics, 44(3), 837–845. https://doi.org/10.2307/2531595.
Dobie, S. A., Baldwin, L. M., Rosenblatt, R. A., Fordyce, M. A., & Andrilla, C. H. (1998). How well do birth certificates describe the pregnancies they report? The Washington State experience with low-risk pregnancies. Maternal and Child Health Journal, 2(3), 145–154.
Dodds, L., Fell, D. B., Shea, S., Armson, B. A., Allen, A. C., & Bryson, S. (2011). The role of prenatal, obstetric and neonatal factors in the development of autism. Journal of Autism and Developmental Disorders, 41(7), 891–902.
Falah, N., Torday, J., Quinney, S. K., & Haas, D. M. (2015). Estriol review: Clinical applications and potential biomedical importance. Clinical Research and Trials. https://doi.org/10.15761/crt.1000109.
Gillon, T. E. R., Pels, A., Dadelszen, P. V., Macdonell, K., & Magee, L. A. (2014). Hypertensive disorders of pregnancy: A systematic review of international clinical practice guidelines. PLoS ONE. https://doi.org/10.1371/journal.pone.0113715.
Goebelsmann, U., & Jaffe, R. B. (1971). Oestriol metabolism in pregnant women. Acta Endocrinologica, 66(4), 679–693.
Goines, P. E., Croen, L. A., Braunschweig, D., Yoshida, C. K., Grether, J., Hansen, R., et al. (2011). Increased midgestational IFN-γ, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Molecular Autism, 2, 13. https://doi.org/10.1186/2040-2392-2-13.
Guller, S., Bulletti, C., Biener, A., & Gurpide, E. (1984). Relative distribution of estrone, estradiol and estriol between fetal and maternal perfusates during perfusions of human term placentas with labelled C19 precursors. Journal of Steroid Biochemistry, 20(4B), 975–979.
Hammond, G. L. (2011). Diverse roles for sex hormone-binding globulin in reproduction. Biology of Reproduction, 85(3), 431–441. https://doi.org/10.1095/biolreprod.111.092593.
Helzlsouer, K. J., Alberg, A. J., Gordon, G. B., Longcope, C., Bush, T. L., Hoffman, S. C., et al. (1995). Serum gonadotropins and steroid hormones and the development of ovarian cancer. JAMA, 274(24), 1926–1930.
Hisle-Gorman, E., Susi, A., Stokes, T., Gorman, G., Erdie-Lalena, C., & Nylund, C. M. (2018). Prenatal, perinatal, and neonatal risk factors of autism spectrum disorder. Pediatric Research, 84(2), 190–198. https://doi.org/10.1038/pr.2018.23.
Holl, K., Lundin, E., Kaasila, M., Grankvist, K., Afanasyeva, Y., Hallmans, G., et al. (2008). Effect of long-term storage on hormone measurements in samples from pregnant women: The experience of the Finnish Maternity Cohort. Acta Oncologica (Stockholm, Sweden), 47(3), 406–412. https://doi.org/10.1080/02841860701592400.
Howland, M. A., Sandman, C. A., & Glynn, L. M. (2017). Developmental origins of the human hypothalamic-pituitary-adrenal axis. Expert Review of Endocrinology & Metabolism, 12(50), 321–339. https://doi.org/10.1080/17446651.2017.1356222.
Ishimoto, H., & Jaffe, R. B. (2011). Development and function of the human fetal adrenal cortex: A key component in the feto-placental unit. Endocrine Reviews, 32(3), 317–355. https://doi.org/10.1210/er.2010-0001.
Jobe, S. O., Tyler, C. T., & Magness, R. R. (2013). Aberrant synthesis, metabolism, and plasma accumulation of circulating estrogens and estrogen metabolites in preeclampsia implications for vascular dysfunction. Hypertension (Dallas, Tex.: 1979), 61(2), 480–487. https://doi.org/10.1161/hypertensionaha.111.201624.
Jones, K. L., Croen, L. A., Yoshida, C. K., Heuer, L., Hansen, R., Zerbo, O., et al. (2017). Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation. Molecular Psychiatry, 22(2), 273–279. https://doi.org/10.1038/mp.2016.77.
Kallen, C. B. (2004). Steroid hormone synthesis in pregnancy. Obstetrics and Gynecology Clinics of North America, 31(4), 795–816. https://doi.org/10.1016/j.ogc.2004.08.009.
Kinnunen, T. I., Luoto, R., Gissler, M., Hemminki, E., & Hilakivi-Clarke, L. (2004). Pregnancy weight gain and breast cancer risk. BMC Womens Health, 4(1), 7.
Krakowiak, P., Walker, C. K., Bremer, A. A., Baker, A. S., Ozonoff, S., Hansen, R. L., et al. (2012). Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics, 129(5), e1121–e1128. https://doi.org/10.1542/peds.2011-2583.
Lawrence, J. M., Contreras, R., Chen, W., & Sacks, D. A. (2008). Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care, 31(5), 899–904. https://doi.org/10.2337/dc07-2345.
Lumey, L. H. (1998). Prenatal oestrogens and breast cancer. Paediatric and Perinatal Epidemiology, 12(4), 361–365.
Magness, R. R., & Rosenfeld, C. R. (1989). Local and systemic estradiol-17 beta: effects on uterine and systemic vasodilation. The American Journal of Physiology, 256(4 Pt 1), E536–E542. https://doi.org/10.1152/ajpendo.1989.256.4.E536.
Malone, F. D., Canick, J. A., Ball, R. H., Nyberg, D. A., Comstock, C. H., Bukowski, R., … First- and second-trimester evaluation of risk (FASTER) research consortium. (2005). First-trimester or second-trimester screening, or both, for Down’s syndrome. The New England Journal of Medicine, 353(19), 2001–2011. https://doi.org/10.1056/nejmoa043693
Martin, J. N., & Cowan, B. D. (1990). Biochemical assessment and prediction of gestational well-being. Obstetrics and Gynecology Clinics of North America, 17(1), 81–93.
Mesiano, S., & Jaffe, R. B. (1997). Developmental and functional biology of the primate fetal adrenal cortex. Endocrine Reviews, 18(3), 378–403. https://doi.org/10.1210/edrv.18.3.0304.
Moisiadis, V. G., & Matthews, S. G. (2014). Glucocorticoids and fetal programming part 1: Outcomes. Nature Reviews Endocrinology, 10(7), 391–402. https://doi.org/10.1038/nrendo.2014.73.
Montenegro, Y. H. A., Nascimento, D. Q., Assis, T. O., & Santos-Lopes, S. S. D. (2019). The epigenetics of the hypothalamic-pituitary-adrenal axis in fetal development. Annals of Human Genetics, 83(4), 195–213. https://doi.org/10.1111/ahg.12306.
Murphy, V. E., Smith, R., Giles, W. B., & Clifton, V. L. (2006). Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus. Endocrine Reviews, 27(2), 141–169. https://doi.org/10.1210/er.2005-0011.
Nahum Sacks, K., Friger, M., Shoham-Vardi, I., Abokaf, H., Spiegel, E., Sergienko, R., et al. (2016). Prenatal exposure to gestational diabetes mellitus as an independent risk factor for long-term neuropsychiatric morbidity of the offspring. American Journal of Obstetrics and Gynecology, 215(3), 380.e1–380.e7. https://doi.org/10.1016/j.ajog.2016.03.030.
Ng, P. C. (2000). The fetal and neonatal hypothalamic–pituitary–adrenal axis. Archives of Disease in Childhood—Fetal and Neonatal Edition, 82(3), F250–F254. https://doi.org/10.1136/fn.82.3.F250.
Palmer, S. K., Zamudio, S., Coffin, C., Parker, S., Stamm, E., & Moore, L. G. (1992). Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstetrics and Gynecology, 80(6), 1000–1006.
Pepe, G., & Albrecht, E. (1995). Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocrine Reviews, 16(5), 608–645.
Pepe, G. J., Waddell, B. J., & Albrecht, E. D. (1990). Activation of the baboon fetal hypothalamic-pituitary-adrenocortical axis at midgestation by estrogen-induced changes in placental corticosteroid metabolism. Endocrinology, 127(6), 3117–3123. https://doi.org/10.1210/endo-127-6-3117.
Petridou, E., Katsouyanni, K., Hsieh, C. C., Antsaklis, A., & Trichopoulos, D. (1992). Diet, pregnancy estrogens and their possible relevance to cancer risk in the offspring. Oncology, 49(2), 127–132.
Resko, J. A., Pleom, J. G., & Stadelman, H. L. (1975). Estrogens in fetal and maternal plasma of the rhesus monkey. Endocrinology, 97(2), 425–430. https://doi.org/10.1210/endo-97-2-425.
Resnik, R., Killam, A. P., Battaglia, F. C., Makowski, E. L., & Meschia, G. (1974). The stimulation of uterine blood flow by various estrogens. Endocrinology, 94(4), 1192–1196. https://doi.org/10.1210/endo-94-4-1192.
Reynolds, R. M. (2013). Glucocorticoid excess and the developmental origins of disease: Two decades of testing the hypothesis–2012 Curt Richter Award Winner. Psychoneuroendocrinology, 38(1), 1–11. https://doi.org/10.1016/j.psyneuen.2012.08.012.
Shin, Y. Y., Jeong, J. S., Park, M.-N., Lee, J.-E., An, S.-M., Cho, W.-S., et al. (2018). Regulation of steroid hormones in the placenta and serum of women with preeclampsia. Molecular Medicine Reports, 17(2), 2681–2688. https://doi.org/10.3892/mmr.2017.8165.
Simpson, E. R., & MacDonald, P. C. (1981). Endocrine physiology of the placenta. Annual Review of Physiology, 43, 163–188. https://doi.org/10.1146/annurev.ph.43.030181.001115.
Smith, K. R. (2019). Pedigree and population resource: Utah population database. Retrieved from https://healthcare.utah.edu/huntsmancancerinstitute/research/updb/
Spratt, E. G., Nicholas, J. S., Brady, K. T., Carpenter, L. A., Hatcher, C. R., Meekins, K. A., et al. (2012). Enhanced cortisol response to stress in children in autism. Journal of Autism and Developmental Disorders, 42(1), 75–81. https://doi.org/10.1007/s10803-011-1214-0.
Strauss, J., Barbieri, R., & Gargiulo, A. (2018). Yen & Jaffe’s reproductive endocrinology (8th ed.). Philadelphia, PA: Elsevier. Retrieved from https://www.elsevier.com/books/yen-and-jaffes-reproductive-endocrinology/9780323479127
Taylor, J. L., & Corbett, B. A. (2014). A review of rhythm and responsiveness of cortisol in individuals with autism spectrum disorders. Psychoneuroendocrinology, 49, 207–228. https://doi.org/10.1016/j.psyneuen.2014.07.015.
Tomarken, A. J., Han, G. T., & Corbett, B. A. (2015). Temporal patterns, heterogeneity, and stability of diurnal cortisol rhythms in children with autism spectrum disorder. Psychoneuroendocrinology, 62, 217–226.
Tordjman, S., Anderson, G. M., Kermarrec, S., Bonnot, O., Geoffray, M.-M., Brailly-Tabard, S., et al. (2014). Altered circadian patterns of salivary cortisol in low-functioning children and adolescents with autism. Psychoneuroendocrinology, 50, 227–245. https://doi.org/10.1016/j.psyneuen.2014.08.010.
Tulchinsky, D., & Hobel, C. J. (1973). Plasma human chorionic gonadotropin, estrone, estradiol, estriol, progesterone, and 17α-hydroxyprogesterone in human pregnancy. American Journal of Obstetrics and Gynecology, 117(7), 884–893.
Tulchinsky, D., & Korenman, S. G. (1971). The plasma estradiol as an index of fetoplacental function. Journal of Clinical Investigation, 50(7), 1490–1497.
van de Beek, C., Thijssen, J. H. H., Cohen-Kettenis, P. T., van Goozen, S. H. M., & Buitelaar, J. K. (2004). Relationships between sex hormones assessed in amniotic fluid, and maternal and umbilical cord serum: What is the best source of information to investigate the effects of fetal hormonal exposure? Hormones and Behavior, 46(5), 663–669. https://doi.org/10.1016/j.yhbeh.2004.06.010.
Walsh, S. W., Wolf, R. C., & Robinson, J. A. (1979). Estrogens in the uteroovarian, uterine, and peripheral plasma in pregnant rhesus monkeys’. Biology of Reproduction, 20, 606–610.
Wang, C., Geng, H., Liu, W., & Zhang, G. (2017). Prenatal, perinatal, and postnatal factors associated with autism: A meta-analysis. Medicine (Baltimore), 96(18), e6696. https://doi.org/10.1097/MD.0000000000006696.
Watterberg, Kristi L. (2004). Adrenocortical function and dysfunction in the fetus and neonate. Seminars in Neonatology: SN, 9(1), 13–21. https://doi.org/10.1016/j.siny.2003.08.003.
Watterberg, K. L., Scott, S. M., & Naeye, R. L. (1997). Chorioamnionitis, cortisol, and acute lung disease in very low birth weight infants. Pediatrics, 99(2), E6.
Windham, G. C., Lyall, K., Anderson, M., & Kharrazi, M. (2016). Autism spectrum disorder risk in relation to maternal mid-pregnancy serum hormone and protein markers from prenatal screening in California. Journal of Autism and Developmental Disorders, 46(2), 478–488. https://doi.org/10.1007/s10803-015-2587-2.
Yeargin-Allsopp, M., Rice, C., Karapurkar, T., Doernberg, N., Boyle, C., & Murphy, C. (2003). Prevalence of autism in a US metropolitan area. JAMA, 289(1), 49–55.
Zerbo, O., Traglia, M., Yoshida, C., Heuer, L. S., Ashwood, P., Delorenze, G. N., et al. (2016). Maternal mid-pregnancy C-reactive protein and risk of autism spectrum disorders: The early markers for autism study. Translational Psychiatry, 6(4), e783. https://doi.org/10.1038/tp.2016.46.
Zinke, K., Fries, E., Kliegel, M., Kirschbaum, C., & Dettenborn, L. (2010). Children with high-functioning autism show a normal cortisol awakening response (CAR). Psychoneuroendocrinology, 35(10), 1578–1582. https://doi.org/10.1016/j.psyneuen.2010.03.009.
Acknowledgments
We thank the Utah FASTER study participants whose contributions were essential for the success of this study. We appreciate the unique collaboration provided across the University of Utah, Intermountain Healthcare, Utah Registry of Autism and Developmental Disabilities, Utah Department of Health, Utah State Board of Education, and the Pedigree and Population Resource (funded by the Huntsman and Intermountain Healthcare Cancer Foundation).
Author information
Authors and Affiliations
Contributions
DBA and AVB designed and performed the research, analyzed data, and wrote the manuscript with input from other authors. MSE, EASC, KRS and AF designed and performed the research. PB and KC designed and performed the research and completed serum analyses. HC designed and performed the research and analyzed the data. TR and WW designed and performed the research and wrote the manuscript with input from the other authors.
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Utah Registry of Autism and Developmental Disabilities Oversight Committee, Utah State Office of Education, and the Institutional Review Boards of the University of Utah, Intermountain Healthcare, Utah Department of Health, and Resource for Genetic and Epidemiologic Research Review Committee, which is an oversight body that regulates Utah Population Database access and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dr. Rayner is now completing his psychiatry residency at the University of Nevada, Reno.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bilder, D.A., Esplin, M.S., Coon, H. et al. Early Second Trimester Maternal Serum Steroid-Related Biomarkers Associated with Autism Spectrum Disorder. J Autism Dev Disord 49, 4572–4583 (2019). https://doi.org/10.1007/s10803-019-04162-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-019-04162-2