Abstract
Wood-derived biochar is an attractive material for supercapcitor electrodes due to its natural hierarchical structure. To improve the conductivity of biochar, graphene oxide is electrophoretically deposited, followed by electrochemical reduction. The conductivity can be controlled by either changing the amount of reduced graphene oxide loading by using different concentrations of graphene oxide suspension or by reaching different reduction states by varying the electrochemical reduction potential. The pore structure is also investigated to evaluate the microstructure effect on the material’s capacitive behavior. A specific capacitance of 167 F g−1 is achieved after reduced graphene oxide deposition, which is 4.3 times higher than that of biochar without reduced graphene oxide. The reduced graphene oxide-modified biochar electrodes show a high rate capability retention of approximately 90% with current densities from 0.5 to 3.0 A g−1. Additionally, no degradation is observed after 10,000 charging–recharging cycles under 5.0 A g−1.
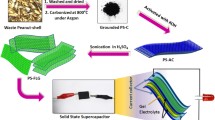
Graphic abstract
As the conductivity of raw biochar is low, it has been modified by reduced graphene oxide through electrophoretic deposition and electrochemical reduction. As a result, the conductivity increases approximately 4 times, but the surface area and pore volume decrease because of pore blockage by graphene. Finally, the reduced graphene oxide-modified biochar electrode exhibits specific capacitance 4.3 times higher than that of raw biochar. The rate capability has also been improved due to the enhanced electron transportation.












Similar content being viewed by others
References
Gutmann G (1999) Hybrid electric vehicles and electrochemical storage systems-a technology push-pull couple. J Power Sources 84:275–279. https://doi.org/10.1016/S0378-7753(99)00328-6
Burke A (2000) Ultracapacitors: why, how, and where is the technology. J Power Sources 91:37–50. https://doi.org/10.1016/S0378-7753(00)00485-7
Jang J, Oh S (2004) Complex capacitance analysis of porous carbon electrodes for electric double-layer capacitors. J Electrochem Soc 151:A571–A577. https://doi.org/10.1149/1.1647572
Chang K, Hu C (2004) Oxidative synthesis of RuOx·nH2O with ideal capacitive characteristics for supercapacitors. J Electrochem Soc 151:A958–A964. https://doi.org/10.1149/1.1755591
Yuan C, Gao B, Shen L, Yang S, Hao L, Lu X, Zhang F, Zhang X (2011) Hierarchically structured carbon-based composites: design, synthesis and their application in electrochemical capacitors. Nanoscale 3:529–545. https://doi.org/10.1039/C0NR00423E
Wang C, Liu T (2017) Nori-based N, O, S, Cl co-doped carbon materials by chemical activation of ZnCl2 for supercapacitor. J Alloys Compd 696:42–50. https://doi.org/10.1016/j.jallcom.2016.11.206
Zhang D, Miao M, Niu H, Wei Z (2014) Core-spun carbon nanotube yarn supercapacitors for wearable electronic textiles. ACS Nano 8:4571–4579. https://doi.org/10.1021/nn5001386
Miller J, Outlaw R, Holloway B (2010) Graphene double-layer capacitor with ac line-filtering performance. Science 329:1637–1639. https://doi.org/10.1126/science.1194372
Kalderis D, Bethanis S, Paraskeva P, Diamadopoulos E (2008) Production of activated carbon from bagasse and rice husk by a single-stage chemical activation method at low retention times. Bioresour Technol 99:6809–6816. https://doi.org/10.1016/j.biortech.2008.01.041
Qiu Z, Wang Y, Bi X, Zhou T, Zhou J, Zhao J et al (2018) Biochar-based carbons with hierarchical micro-meso-macro porosity for high rate and long cycle life supercapacitors. J Power Sources 376:82–90. https://doi.org/10.1016/j.jpowsour.2017.11.077
Guo S, Peng J, Li W, Yang K, Zhang L, Zhang S et al (2009) Effects of CO2 activation on porous structures of coconut shell-based activated carbons. Appl Surf Sci 255:8443–8449. https://doi.org/10.1016/j.apsusc.2009.05.150
Yin H, Lu B, Xu Y et al (2014) Harvesting capacitive carbon by carbonization of waste biomass in molten salts. Environ Sci Technol 48:8101–8108. https://doi.org/10.1021/es501739v
Lehmann J, Rilling M, Thies J, Masiello C (2011) Biochar effects on soil biota—A review. Soil Biol Biochem 43:1812–1836. https://doi.org/10.1016/j.soilbio.2011.04.022
Funabashi T, Mizuno J, Sato M, Kitajima M, Matsuura M, Shoji S (2013) Film of lignocellulosic carbon material for self-supporting electrodes in electric double-layer capacitors. APL Mater 1:032104. https://doi.org/10.1063/1.4820430
Jin H, Wang X, Gu Z, Polin J (2013) Carbon materials from high ash biochar for supercapacitor and improvement of capacitance with HNO3 surface oxidation. J Power Sources 236:285–292. https://doi.org/10.1016/j.jpowsour.2013.02.088
Wan C, Jiao Y, Li J (2016) Core-shell composite of wood-derived biochar supported MnO2 nanosheets for supercapacitor applications. RSC Adv 6:64811–64817. https://doi.org/10.1039/C6RA12043A
Jiang J, Zhang L, Wang X, Holm N et al (2013) Highly ordered macroporous woody biochar with ultra-high carbon content as supercapacitor electrodes. Electrochim Acta 113:481–489. https://doi.org/10.1016/j.electacta.2013.09.121
Liu M, Kong L, Zhang P, Luo Y et al (2012) Porous wood carbon monolith for high-performance supercapacitors. Electrochim Acta 60:443–448. https://doi.org/10.1016/j.electacta.2011.11.100
Chen H, Liu D, Shen Z, Bao B, Zhao S, Wu L (2015) Functional biomass carbons with hierarchical porous structure for supercapacitor electrode materials. Electrochim Acta 180:241–251. https://doi.org/10.1016/j.electacta.2015.08.133
Kuratani K, Okuno K, Iwaki T, Kato M, Takeichi N, Miyuki T, Awazu T, Majima M, Sakai T (2011) Converting rice husk activated carbon into active material for capacitor using three-dimensional porous current collector. J Power Sources 196:10788–10790. https://doi.org/10.1016/j.jpowsour.2011.09.001
Gupta R, Dubey M, Kharel P, Gu Z, Fan Q (2015) Biochar activated by oxygen plasma for supercapacitors. J Power Sources 274:1300–1305. https://doi.org/10.1016/j.jpowsour.2014.10.169
Genovese M, Jiang J, Lian K, Holm N (2015) High capacitive performance of exfoliated biochar nanosheets from biomass waste corn cob. J Mater Chem A 3:2903–2913. https://doi.org/10.1039/C4TA06110A
Nirmalraj P, Lutz T, Kumar S, Duesberg G, Boland J (2011) Nanoscale mapping of electrical resistivity and connectivity in graphene strips and networks. Nano Lett 11:16–22. https://doi.org/10.1021/nl101469d
Xu B, Yue S, Sui Z, Zhang X, Hou S, Cao G et al (2011) What is the choice for supercapacitors: graphene or graphene oxide? Energy Environ Sci 4:2826–2830. https://doi.org/10.1039/C1EE01198G
Abdul G, Zhu X, Chen B (2017) Structural characteristics of biochar-graphene nanosheet composites and their adsorption performance for phthalic acid esters. Chem Eng J 319:9–20. https://doi.org/10.1016/j.cej.2017.02.074
Wang C, Li J, Sun S, Zhao F, Jiang B, Huang Y (2016) Electrophoretic deposition of graphene oxide on continuous carbon fibers for reinforcement of both tensile and interfacial strength. Compos Sci Technol 135:46–53. https://doi.org/10.1016/j.compscitech.2016.07.009
Lu T, Pan L, Li H, Nie C et al (2011) Reduced graphene oxide–carbon nanotubes composite films by electrophoretic deposition method for supercapacitors. J Electroanal Chem 661:270–273. https://doi.org/10.1016/j.jelechem.2011.07.042
Sing K (1985) Reporting physisorption data for gas/solid system with special reference to the determination of surface area and porosity. Pure Appl Chem 57:603–619
Huang J, Xu Z, Aboualic S, Garakani M, Kim J (2016) Porous graphene oxide/carbon nanotube hybrid films as interlayer for lithium-sulfur batteries. Carbon 99:624–632. https://doi.org/10.1016/j.carbon.2015.12.081
Luo Q, Ge R, Kang S, Qin L, Ling G, Li X (2018) Fabrication mechanism and photocatalytic activity for a novel Graphene oxide hybrid functionalized with tetrakis-(4-hydroxylphenyl) porphyrin and 1-pyrenesulfonic acid. Appl Surf Sci 427:15–23. https://doi.org/10.1016/j.apsusc.2017.08.152
Cai Z, Jiang C, Zhang XF, Zhang YS, Liang L (2018) Lignin-based biochar/graphene oxide composites as supercapacitor electrode materials. Mater Sci Eng 359:012046. https://doi.org/10.1088/1757-899X/359/1/012046
Ghosh A, Lee Y (2012) Carbon-based electrochemical capacitors. Chemsuschem 5:480–499. https://doi.org/10.1002/cssc.201100645
Wan C, Jiao Y, Li J (2016) Core–shell composite of wood-derived biochar supported MnO2 nanosheets for supercapacitor applications. RSC Adv 6:64811–64817. https://doi.org/10.1039/C6RA12043A
Wan C, Li J (2016) Wood-derived biochar supported polypyrrole nanoparticles as a free-standing supercapacitor electrode. RSC Adv 6:86006–86011. https://doi.org/10.1039/C6RA17044G
Toh S, Loh K, Kamarudin S, Daud W (2016) The impact of electrochemical reduction potentials on the electrocatalytic activity of graphene oxide toward the oxygen reduction reaction in an alkaline medium. Electrochim Acta 199:194–203. https://doi.org/10.1016/j.electacta.2016.03.103
Toh S, Loh K, Kamarudin S, Daud W (2014) Graphene production via electrochemical reduction of graphene oxide: synthesis and characterization. Chem Eng J 251:422–434. https://doi.org/10.1016/j.cej.2014.04.004
Zeng F, Sun Z, Sang X, Diamond D, Lau K, Liu X, Su D (2011) In situ one-step electrochemcial preparation of graphene oxide nanosheet-modified electrode for biosensor. Chemsuschem 4:1587–1591. https://doi.org/10.1002/cssc.201100319
Acknowledgements
XRD measurement is supported by instrumental and analysis research center, Shanghai University. This work is supported by the Shanghai Sailing Program (Grant No. 16YF1404500).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rui, B., Yang, M., Zhang, L. et al. Reduced graphene oxide-modified biochar electrodes via electrophoretic deposition with high rate capability for supercapacitors. J Appl Electrochem 50, 407–420 (2020). https://doi.org/10.1007/s10800-020-01397-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-020-01397-1