Abstract
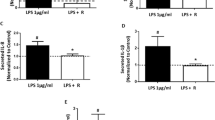
Upregulation of chemokine CX3CL1 and its receptor CX3CR1 occurs in the diabetic human placenta. Metformin, an insulin-sensitizing biguanide, is used in the therapy of diabetic pregnancy. By preventing the activation of NF-κB, metformin exhibits anti-inflammatory properties. We examined the influence of hyperglycemia (25 mmol/L glucose; HG group; N = 36) on metformin-mediated effects on CX3CL1 and TNF-α production by placental lobules perfused extracorporeally. Additionally, CX3CR1 expression and contents of CX3CR1, TNF-α receptor 1 (TNFR1), and NF-κB proteins in the placental tissue were evaluated. Placentae perfused under normoglycemia (5 mmol/L glucose; NG group; N = 36) served as the control. Metformin (2.5 and 5.0 mg/L; subgroups B and C) lowered the production of CX3CL1 and TNF-α in a dose-dependent and time-dependent manner. Hyperglycemia did not weaken the strength of these metformin effects. Moreover, CX3CL1 levels after perfusion with 5.0 mg/L metformin were reduced by 33.28 and 33.83% (at 120 and 150 min, respectively) in the HG-C subgroup versus 24.98 and 23.66% in the NG-C subgroup, which indicated an augmentation of the metformin action over time in hyperglycemia. CX3CR1 expression was significantly higher in the HG-B and HG-C subgroups compared to that in the NG-B and NG-C subgroups. Increased CX3CR1 protein content in the placental lysates was observed in subgroups B and C. The two higher metformin concentrations significantly decreased the levels of NF-κBp65 protein content in both groups. However, the decrease was significantly stronger in hyperglycemia. TNFR1 upregulation in the HG group was not affected by metformin. Further studies on metformin therapy during pregnancy are needed, including safety issues.





Similar content being viewed by others
Abbreviations
- ADAMs:
-
Desintegrin and metalloproteinases
- ADAM-10:
-
Disintegrin and metalloproteinase (ADAM) 10
- ADAM-17:
-
Desintegrin and metalloproteinase (ADAM) 17
- Act:
-
Serine/threonine kinase Akt (protein kinase B, PKB)
- ALT:
-
Alanine aminotransferase
- AMPK:
-
Adenosine 5’- monophosphate(AMP)-activated protein kinase
- AST:
-
Aspartate aminotransferase
- BDNF:
-
Brain-derived neurotrophic factor
- BLC:
-
Chemokine CXCL13
- CCL4, CCL7, CCL14:
-
C-C motif chemokines: ligand 4, 7, and 14, respectively
- CPU:
-
Central processing unit
- CX3CL1:
-
C-X3-C motif chemokine ligand 1(fractalkine, neurotactin)
- CX3CR1:
-
Chemokine CX3CL1 receptor 1
- DMSO:
-
Dimethyl sulfoxide
- ELISA:
-
Enzyme-linked immunosorbent assay
- ENA-78:
-
Chemokine CXCL5
- FGF-4:
-
Fibroblast growth factor 4
- G-CSF:
-
Granulocyte colony-stimulating factor
- GDM:
-
Gestational diabetes mellitus
- GIT:
-
glucose impaired tolerance, prediabetes
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- HG:
-
High glucose
- HUVECs:
-
Human umbilical vein endothelial cells
- IFN-γ :
-
Interferon gamma
- IKK:
-
IκB kinase complex
- IL:
-
Interleukin
- initCX3CL1:
-
Initial concentrations of CX3CL1
- initTNF-α :
-
Initial concentrations of TNF-α
- IP-10:
-
Chemokine CXCL10 (interferon gamma-induced protein 10; small inducible cytokine B10)
- JAK/STAT:
-
Janus kinase/signal transducers and activators of transcription
- LPS:
-
Lipopolysaccharide
- MCP-1-3:
-
Monocyte chemotactic proteins
- MDC:
-
Macrophage-derived chemokine
- MIP-1α, MIP-1β, MIP-1δ :
-
Macrophage inflammatory proteins 1: alpha, beta, delta
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NG:
-
Normal glucose
- NIK:
-
NF-κB-inducing kinase
- NK:
-
Natural killer cells
- PARC:
-
Pulmonary and activation-regulated chemokine (chemokine CCL18)
- PDGF:
-
Platelet-derived growth factor
- PDK1:
-
Pyruvate dehydrogenase kinase 1 (pyruvate dehydrogenase [acetyl-transferring] kinase isozyme 1)
- PBS:
-
Phosphate-buffered saline
- PI3-kinase:
-
Phosphoinositide 3-kinase (phosphatidylinositol-4,5-bisphosphate 3-kinase)
- pO2 :
-
Oxygen partial pressure
- RANTES:
-
Regulated on activation, normal T-cell expressed and secreted (chemokine CCL5)
- SCF:
-
Stem cell factor
- TARC:
-
Thymus- and activation-regulated chemokine (chemokine CCL17)
- TGF-β :
-
Transforming growth factor β
- TIMP-1-2:
-
Tissue inhibitors of metalloproteinases
- TNFRSF1A:
-
Tumor necrosis factor receptor superfamily member 1A (tumor necrosis factor receptor 1; CD120a)
- TNF-α, TNF-β :
-
Tumor necrosis factors: alpha, beta
- TPO:
-
Thyroid peroxidase
- VEGF:
-
Vascular endothelial growth factor
- V/EVTI:
-
Vascular/extravascular tissular index
References
Wedekind, L., and L. Belkacemi. 2016. Altered cytokine network in gestational diabetes mellitus affects maternal insulin and placental-fetal development. Journal of Diabetes and Its Complications 30: 1393–1400.
Desoye, G., and S. Hauguel-de Mouzon. 2007. The human placenta in gestational diabetes mellitus. The insulin and cytokine network. Diabetes Care 30 (Suppl 2): S120–S126 Erratum in: Diabetes Care 2007; 30: 3154.
Navarro-González, J.F., and C. Mora-Fernández. 2011. Inflammatory pathways. In Diabetes and the kidney. Contributions to nephrology, ed. K.N. Lai and S.C.W. Tang, vol. 170, 113–123. Basel: Karger.
Dragomir, E., I. Manduteanu, M. Calin, A.M. Gan, D. Stan, R.R. Koenen, et al. 2008. High glucose conditions induce upregulation of fractalkine and monocyte chemotactic protein-1 in human smooth muscle cells. Thrombosis and Haemostasis 100: 1155–1165.
Szukiewicz, D., J. Kochanowski, M. Pyzlak, G. Szewczyk, A. Stangret, and T.K. Mittal. 2013. Fractalkine (CX3CL1) and its receptor CX3CR1 may contribute to increased angiogenesis in diabetic placenta. Mediators of Inflammation 2013: 437576.
Yao, L., O. Herlea-Pana, J. Heuser-Baker, Y. Chen, and J. Barlic-Dicen. 2014. Roles of the chemokine system in development of obesity, insulin resistance, and cardiovascular disease. Journal of Immunology Research 2014: 11.
Das, A., and S. Mukhopadhyay. 2011. The evil axis of obesity, inflammation and type-2 diabetes. Endocrine, Metabolic & Immune Disorders Drug Targets 11: 23–31.
Apostolakis, S., and D. Spandidos. 2013. Chemokines and atherosclerosis: Focus on the CX3CL1/CX3CR1 pathway. Acta Pharmacologica Sinica 34: 1251–1256.
Bergmann, K., and G. Sypniewska. 2014. Secreted frizzled-related protein 4 (SFRP4) and fractalkine (CX3CL1)—Potential new biomarkers for β-cell dysfunction and diabetes. Clinical Biochemistry 47: 529–532.
Ebert, T., J. Hindricks, S. Kralisch, U. Lossner, B. Jessnitzer, J. Richter, et al. 2014. Serum levels of fractalkine are associated with markers of insulin resistance in gestational diabetes. Diabetic Medicine 31: 1014–1017.
Shah, R., S.M. O’Neill, C. Hinkle, J. Caughey, S. Stephan, E. Lynch, et al. 2015. Metabolic effects of CX3CR1 deficiency in diet-induced obese mice. PLoS One 10: e0138317.
Fiorentino, T.V., A. Prioletta, P. Zuo, and F. Folli. 2013. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Current Pharmaceutical Design 19: 5695–5703.
Mrizak, I., O. Grissa, B. Henault, M. Fekih, A. Bouslema, I. Boumaiza, et al. 2014. Placental infiltration of inflammatory markers in gestational diabetic women. General Physiology and Biophysics 33: 169–176.
Myatt, L. 1992. Control of vascular resistance in the human placenta. Placenta 13: 329–341.
Domingueti, C.P., L.M. Dusse, M.D. Carvalho, L.P. de Sousa, K.B. Gomes, and A.P. Fernandes. 2016. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. Journal of Diabetes and Its Complications 30: 738–745.
Kervancioglu Demirci, E., L.A. Salamonsen, and M. Gauster. 2016. The role of CX3CL1 in fetal-maternal interaction during human gestation. Cell Adhesion and Migration 10: 189–196.
Dimitriadis, E., G. Nie, N.J. Hannan, P. Paiva, and L.A. Salamonsen. 2010. Local regulation of implantation at the human fetal-maternal interface. International Journal of Developmental Biology 54: 313–322.
Bowen, J.M., L. Chamley, M.D. Mitchell, and J.A. Keelan. 2002. Cytokines of the placenta and extra-placental membranes: Biosynthesis, secretion and roles in establishment of pregnancy in women. Placenta 23: 239–256.
Taki, A., M. Abe, M. Komaki, K. Oku, S. Iseki, S. Mizutani, et al. 2012. Expression of angiogenesis-related factors and inflammatory cytokines in placenta and umbilical vessels in pregnancies with preeclampsia and chorioamnionitis/funisitis. Congenital Anomalies (Kyoto) 52: 97–103.
Bazan, J.F., K.B. Bacon, G. Hardiman, W. Wang, K. Soo, D. Rossi, et al. 1997. A new class of membrane-bound chemokine with a CX3C motif. Nature 385: 640–644.
Pan, Y., C. Lloyd, H. Zhou, S. Dolich, J. Deeds, J.A. Gonzalo, et al. 1997. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature 387: 611–617 Erratum in: Nature 1997; 389 100.
Kim, K.W., A. Vallon-Eberhard, E. Zigmond, J. Farache, E. Shezen, G. Shakhar, et al. 2011. In vivo structure/function and expression analysis of the CX3C chemokine fractalkine. Blood 118: e156–e167.
Matsumiya, T., K. Ota, T. Imaizumi, H. Yoshida, H. Kimura, and K. Satoh. 2010. Characterization of synergistic induction of CX3CL1/fractalkine by TNF-alpha and IFN-gamma in vascular endothelial cells: An essential role for TNF-alpha in post-transcriptional regulation of CX3CL1. Journal of Immunology 184: 4205–4214.
Sindhu, S., N. Akhter, H. Arefanian, A.A. Al-Roub, S. Ali, A. Wilson, et al. 2017. Increased circulatory levels of fractalkine (CX3CL1) are associated with inflammatory chemokines and cytokines in individuals with type-2 diabetes. Journal of Diabetes and Metabolic Disorders 15: 15.
Clark, A.K., A.A. Staniland, and M. Malcangio. 2011. Fractalkine/CX3CR1 signaling in chronic pain and inflammation. Current Pharmaceutical Biotechnology 12: 1707–1714.
D’Haese, J.G., I.E. Demir, H. Friess, and G.O. Ceyhan. 2010. Fractalkine/CX3CR1: Why a single chemokine-receptor duo bears a major and unique therapeutic potential. Expert Opinion on Therapeutic Targets 14: 207–219.
Chandrasekar, B., S. Mummidi, R.P. Perla, S. Bysani, N.O. Dulin, F. Liu, et al. 2003. Fractalkine (CX3CL1) stimulated by nuclear factor kappaB (NF-kappaB)-dependent inflammatory signals induces aortic smooth muscle cell proliferation through an autocrine pathway. Biochemical Journal 373: 547–558.
Sung, M.J., D.H. Kim, M. Davaatseren, H.J. Hur, W. Kim, Y.J. Jung, et al. 2010. Genistein suppression of TNF-alpha-induced fractalkine expression in endothelial cells. Cellular Physiology and Biochemistry 26: 431–440.
Cabal-Hierro, L., and P.S. Lazo. 2012. Signal transduction by tumor necrosis factor receptors. Cellular Signalling 24: 1297–1305.
Inzucchi, S.E., R.M. Bergenstal, J.B. Buse, M. Diamant, E. Ferrannini, M. Nauck, et al. 2015. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 38: 140–149.
Marín-Peñalver, J.J., I. Martín-Timón, C. Sevillano-Collantes, and F.J. del Cañizo-Gómez. 2016. Update on the treatment of type 2 diabetes mellitus. World Journal of Diabetes 7: 354–395.
Ijäs, H., M. Vääräsmäki, L. Morin-Papunen, R. Keravuo, T. Ebeling, T. Saarela, et al. 2011. Metformin should be considered in the treatment of gestational diabetes: A prospective randomised study. British Journal of Obstetrics and Gynaecology 118: 880–885.
Zhao, L.P., X.Y. Sheng, S. Zhou, T. Yang, L.Y. Ma, Y. Zhou, et al. 2015. Metformin versus insulin for gestational diabetes mellitus: A meta-analysis. British Journal of Clinical Pharmacology 80: 1224–1234.
Charles, B., R. Norris, X. Xiao, and W. Hague. 2006. Population pharmacokinetics of metformin in late pregnancy. Therapeutic Drug Monitoring 28: 67–72.
Vanky, E., K. Zahlsen, O. Spigset, and S.M. Carlsen. 2005. Placental passage of metformin in women with polycystic ovary syndrome. Fertility and Sterility 83: 1575–1578.
Hattori, Y., K. Suzuki, S. Hattori, and K. Kasai. 2006. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension 47: 1183–1188.
Saisho, Y. 2015. Metformin and inflammation: Its potential beyond glucose-lowering effect. Endocrine Metabolic & Immune Disorders Drug Targets 15: 196–205.
Rena, G., D.G. Hardie, and E.R. Pearson. 2017. The mechanism of action of metformin. Diabetologia 60: 1577–1585.
Szukiewicz, D., M. Pyzlak, G. Szewczyk, A. Stangret, S. Trojanowski, M. Bachanek, et al. 2017. High glucose level disturbs the resveratrol-evoked curtailment of CX3CL1/CX3CR1 signaling in human placental circulation. Mediators of Inflammation 2017: 9853108.
Schneider, H., K.H. Möhlen, J.C. Challier, and J. Dancis. 1979. Transfer of glutamic acid across the human placenta perfused in vitro. British Journal of Obstetrics and Gynaecology 86: 299–306.
Szukiewicz, D., J. Kochanowski, T.K. Mittal, M. Pyzlak, G. Szewczyk, and K. Cendrowski. 2014. CX3CL1 (fractalkine) and TNFα production by perfused human placental lobules under normoxic and hypoxic conditions in vitro: The importance of CX3CR1 signaling. Inflammation Research 63: 179–189.
Kajbaf, F., M.E. De Broe, and J.D. Lalau. 2016. Therapeutic concentrations of metformin: A systematic review. Clinical Pharmacokinetics 55: 439–459.
Scheen, A.J. 2013. Pharmacokinetic considerations for the treatment of diabetes in patients with chronic kidney disease. Expert Opinion on Drug Metabolism &Toxicology 9: 529–550.
Hoesel, B., and J.A. Schmid. 2013. The complexity of NF-κB signaling in inflammation and cancer. Molecular Cancer 12: 1–15.
Ryu, J., C.W. Lee, K.H. Hong, J.A. Shin, S.H. Lim, C.S. Park, et al. 2008. Activation of fractalkine/CX3CR1 by vascular endothelial cells induces angiogenesis through VEGF-A/KDR and reverses hindlimb ischaemia. Cardiovascular Research 78: 333–340.
Szukiewicz, D., M. Wojciechowska, A. Bilska, A. Stangret, G. Szewczyk, T.K. Mittal, et al. 2015. Aspirin action in endothelial cells: Different patterns of response between chemokine CX3CL1/CX3CR1 and TNF-α/TNFR1 signaling pathways. Cardiovascular Drugs and Therapy 29: 219–229.
Yang, X.P., S. Mattagajasingh, S. Su, G. Chen, Z. Cai, K. Fox-Talbot, et al. 2007. Fractalkine upregulates intercellular adhesion molecule-1 in endothelial cells through CX3CR1 and the Jak Stat5 pathway. Circulation Research 101: 1001–1008.
Szukiewicz, D., A. Szukiewicz, D. Maslinska, G. Szewczyk, and M. Watroba. 2003. Mast cell-derived vascular endothelial growth factor (VEGF) and microvascular density in diabetic placentae. Inflammation Research 52 (Supplement 1): S9–S10.
Szukiewicz, D., A. Szukiewicz, D. Maslinska, and M.W. Markowski. 1999. Placental mast cells (MC) and histamine (HA) in pregnancy complicated by diabetes class C—Relation to the development of villous microvessels. Placenta 20 (Supplement 1): 503–510.
Huppertz, B., E. Abe, P. Murthi, T. Nagamatsu, D. Szukiewicz, and C. Salafia. 2007. Placental angiogenesis, maternal and fetal vessels—A workshop report. Placenta 28 (Supplement A): S94–S96.
Gui, J., Q. Liu, and L. Feng. 2013. Metformin vs insulin in the management of gestational diabetes: A meta-analysis. PLoS One 8: e64585.
Arshad, R., S. Khanam, F. Shaikh, and N. Karim. 2017. Feto-maternal outcomes and glycemic control in metformin versus insulin treated gestational diabetics. Pakistan Journal of Medical Sciences 33: 1182–1187.
Serhan, C.N. 2017. Treating inflammation and infection in the 21st century: New hints from decoding resolution mediators and mechanisms. FASEB Journal 31: 1273–1288.
Lattenist, L., P. Ochodnicky, M. Ahdi, N. Claessen, J.C. Leemans, S.C. Satchell, et al. 2016. Renal endothelial protein C receptor expression and shedding during diabetic nephropathy. Journal of Thrombosis and Haemostasis 14: 1171–1182.
Hurst, L.A., R.A. Bunning, B. Sharrack, and M.N. Woodroofe. 2012. siRNA knockdown of ADAM-10, but not ADAM-17, significantly reduces fractalkine shedding following pro-inflammatory cytokine treatment in a human adult brain endothelial cell line. Neuroscience Letters 521: 52–56.
Hyun, B., S. Shin, A. Lee, S. Lee, Y. Song, N.J. Ha, et al. 2013. Metformin down-regulates TNF-α secretion via suppression of scavenger receptors in macrophages. Immune Network 13: 123–132.
Kikuchi, Y., R. Ikee, N. Hemmi, N. Hyodo, T. Saigusa, T. Namikoshi, et al. 2004. Fractalkine and its receptor, CX3CR1, upregulation in streptozotocin-induced diabetic kidneys. Nephron Experimental Nephrology 97: e17–e25.
Cho, J.G., J.J. Song, J. Choi, G.J. Im, H.H. Jung, and S.W. Chae. 2016. The suppressive effects of metformin on inflammatory response of otitis media model in human middle ear epithelial cells. International Journal of Pediatric Otorhinolaryngology 89: 28–32.
Ramana, K.V., B. Friedrich, S. Srivastava, A. Bhatnagar, and S.K. Srivastava. 2004. Activation of nuclear factor-κB by hyperglycemia in vascular smooth muscle cells is regulated by aldose reductase. Diabetes 53: 2910–2920.
Mitchell, S., J. Vargas, and A. Hoffmann. 2016. Signaling via the NFκB system. Wiley Interdisciplinary Reviews: Systems Biology and Medicine 8: 227–241.
Jawerbaum, A., and E. González. 2006. Diabetic pregnancies: The challenge of developing in a pro-inflammatory environment. Current Medicinal Chemistry 13: 2127–2138.
Magee, T.R., M.G. Ross, L. Wedekind, M. Desai, S. Kjos, and L. Belkacemi. 2014. Gestational diabetes mellitus alters apoptotic and inflammatory gene expression of trophoblasts from human term placenta. Journal of Diabetes and Its Complications 28: 448–459.
Ozsoy, H.Z., N. Sivasubramanian, E.D. Wieder, S. Pedersen, and D.L. Mann. 2008. Oxidative stress promotes ligand-independent and enhanced ligand-dependent tumor necrosis factor receptor signaling. Journal of Biological Chemistry 283: 23419–23428.
Fernández-Juárez, G., J.V. Perez, J.L. Fernández, E. Martinez-Martinez, V. Cachofeiro, V. Barrio Lucia, et al. 2017. High levels of circulating TNFR1 increase the risk of all-cause mortality and progression of renal disease in type 2 diabetic nephropathy. Nephrology (Carlton) 22: 354–360.
Schmidt, A., D.M. Morales-Prieto, J. Pastuschek, K. Fröhlich, and U.R. Markert. 2015. Only humans have human placentas: Molecular differences between mice and humans. Journal of Reproductive Immunology 108: 65–71.
Umehara, H., E.T. Bloom, T. Okazaki, Y. Nagano, O. Yoshie, and T. Imai. 2004. Fractalkine in vascular biology: From basic research to clinical disease. Arteriosclerosis, Thrombosis, and Vascular Biology 24: 34–40.
Golovchenko, I., N.L. Goalstone, P. Watson, M. Brownlee, and B. Draznin. 2000. Hyperinsulinemia enhances transcriptional activity of nuclear factor-κB induced by angiotensin II, hyperglycemia, and advanced glycosylation end products in vascular smooth muscle cells. Circulation Research 87: 746–752.
Macedo da Costa, T.H., F.V. Pires da Silva, C.E. Gonçalves Reis, and Casulari L. Augusto. 2014. Improved metabolic response after 16 weeks of calorie-restricted low-glycaemic index diet and metformin in impaired glucose tolerance subjects. Nutricion Hospitalaria 29: 1081–1087.
Dodd, J.M., R.M. Grivell, A.R. Deussen, G. Dekker, J. Louise, and W. Hague. 2016. Metformin and dietary advice to improve insulin sensitivity and promote gestational restriction of weight among pregnant women who are overweight or obese: The GRoW Randomised Trial. BMC Pregnancy and Childbirth 16: 359.
Cvitic, S., G. Desoye, and U. Hiden. 2014. Glucose, insulin, and oxygen interplay in placental hypervascularisation in diabetes mellitus. BioMed Research International 2014: 12.
Li, H.P., X. Chen, and M.Q. Li. 2013. Gestational diabetes induces chronic hypoxia stress and excessive inflammatory response in murine placenta. International Journal of Clinical and Experimental Pathology 6: 650–659.
Spradley, F.T. 2017. Metabolic abnormalities and obesity’s impact on the risk for developing preeclampsia. American Journal of Physiology. Regulatory Integrative and Comparative Physiology 312: R5–R12.
Szukiewicz, D., M. Pyzlak, G. Szewczyk, J. Wejman, and J. Kochanowski. 2017. Hypoxia disturbs the metformin-evoked curtailment of chemokine CX3CL1 (fractalkine) signaling in human umbilical vein endothelial cells (HUVECs). Abstract in: The FASEB Journal 31 (Suppl): 833.9.
Leach, L., A. Taylor, and F. Sciota. 2009. Vascular dysfunction in the diabetic placenta: Causes and consequences. Journal of Anatomy 215: 69–76.
Ainuddin, J.A., N. Karim, S. Zaheer, S.S. Ali, and A.A. Hasan. 2015. Metformin treatment in type 2 diabetes in pregnancy: An active controlled, parallel-group, randomized, open label study in patients with type 2 diabetes in pregnancy. Journal of Diabetes Research 2015: 325851.
De Haes, W., L. Frooninckx, R. Van Assche, A. Smolders, G. Depuydt, J. Billen, et al. 2014. Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2. Proceedings of the National Academy of Sciences of the United States of America 111: E2501–E2509.
Liang, H.L., S.J. Ma, Y.N. Xiao, and H.Z. Tan. 2017. Comparative efficacy and safety of oral antidiabetic drugs and insulin in treating gestational diabetes mellitus: An updated PRISMA-compliant network meta-analysis. Medicine (Baltimore) 96: e7939.
Vanlalhruaii, R. Dasgupta, R. Ramachandran, J.E. Mathews, A. Regi, N. Thomas, et al. 2018. How safe is metformin when initiated in early pregnancy? A retrospective 5-year study of pregnant women with gestational diabetes mellitus from India. Diabetes Research and Clinical Practice 137: 47–55.
Feig, D.S., and R.G. Moses. 2011. Metformin therapy during pregnancy: Good for the goose and good for the gosling too? Diabetes Care 34: 2329–2330.
Rowan, J.A., E.C. Rush, V. Obolonkin, M. Battin, T. Wouldes, and W.M. Hague. 2011. Metformin in gestational diabetes: The offspring follow-up (MiG TOFU): Body composition at 2 years of age. Diabetes Care 34: 2279–2284.
Panchaud, A., V. Rousson, T. Vial, N. Bernard, D. Baud, E. Amar, et al. 2017. Pregnancy outcomes in women on metformin for diabetes or other indications among those seeking teratology information services. British Journal of Clinical Pharmacology 84: 568–578.
Acknowledgements
The creative contribution to the study design provided by Professor Slawomir Maslinski is gratefully acknowledged.
Funding
This study was funded by internal Grant no. 2M2/W1/16, funded by the Medical University of Warsaw, Poland. No additional external funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Szukiewicz, D., Szewczyk, G., Pyzlak, M. et al. Anti-inflammatory Action of Metformin with Respect to CX3CL1/CX3CR1 Signaling in Human Placental Circulation in Normal-Glucose Versus High-Glucose Environments. Inflammation 41, 2246–2264 (2018). https://doi.org/10.1007/s10753-018-0867-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0867-7