Abstract
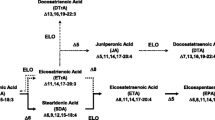
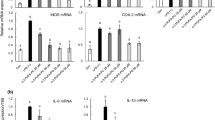
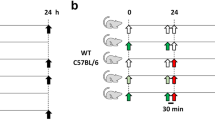
Lysophosphatidylcholines (lysoPCs) have been known to be bioactive lipid mediators, which take part in various biological and pathological processes. In the present study, we examined the anti-inflammatory actions of 2-docosahexaenoyl lysophosphatidylcholine (2-docosahexaenoyl-lysoPC) in vitro as well as in vivo systems. When RAW 264.7 cells were treated with 2-docoshexaenoyl-lysoPC, a concentration-dependent decrease of LPS-induced formation of nitric oxide (NO), tumor necrosis factor alpha (TNF-α), or IL-6 was observed. Additionally, oral administration of 2-docosahexaenoyl-lysoPC was found to inhibit zymosan A-induced plasma leakage dose-dependently in mice with ED50 value of 50 μg/kg and E max value of about 65%. Moreover, mechanistic study revealed that the anti-inflammatory action of 2-docosahexaenoyl-lysoPC seemed to be related largely to LTC4 inhibition, but not PGE2 inhibition. Moreover, 2-(17-hydroperoxydocosahexaneoyl)-lysoPC, intravenously administrated, was more effective than 2-docosahexaenoyl-lysoPC in the inhibition of zymosan A-induced plasma leakage, suggesting that 2-(17-hydroperoxydocosahexaneoyl)-lysoPC, a product from oxygenation of 2-docosahexaenoyl-lysoPC by 15-lipoxygenase (LOX), may be an active metabolite, intimately responsible for anti-inflammatory actions, generated from 2-docosahexaenoyl-lysoPC. In a related study, 2-docosahexaenoyl-lysoPC was found to be more efficient than 1-docosahexaenoyl-lysoPC or docosahexaenoic acid (DHA) as substrate for 15-lipoxygenases such as soybean LOX-1, leukocyte 12/15-LOX, and human 15-LOX-2. Taken altogether, it is suggested that 2-docosahexaenoyl-lysoPC and its oxygenation products may exert anti-inflammatory action after oral administration.










Similar content being viewed by others
Abbreviations
- DHA:
-
docosahexaenoic acid
- 17-HDoHA:
-
17-hydroxydocoxahexaenoic acid
- 1-linoleoyl-lysoPC:
-
1-linoleoyl-lysophosphatidylcholine
- 1-arachidonoyl-lysoPC:
-
1-arachidonoyl-lysophosphatidylcholine
- 1-docosahexaenoyl-lysoPC:
-
1-docosahexaenoyl-lysophosphatidylcholine
- 2-docosahexaenoyl-lysoPC:
-
2-docosahexaenoyl-lysophosphatidylcholine
- LOX:
-
lipoxygenase
- ED50 :
-
50% effective dose
- PLA2 :
-
phospholipase A2
- LTC4 :
-
leukotriene C4
- PGE2 :
-
prostaglandin E2
- IL-6:
-
Interleukin-6
- TNF-α:
-
tumor necrosis factor alpha
- NO:
-
nitric oxide
References
Serhan, C.N., and J. Savill. 2005. Resolution of inflammation: The beginning programs the end. Nature Immunology 6: 1191–1197.
Levy, B.D., C.B. Clish, B. Schmidt, K. Gronert, and C.N. Serhan. 2001. Lipid mediator class switching during acute inflammation: Signals in resolution. Nature Immunology 2(7): 612–619.
Serhan, C.N. 2005. Novel eicosanoid and docosanoid mediators: Resolvins, docosatrienes, and neuroprotectins. Current Opinion in Clinical Nutrition and Metabolic Care 8: 115–121.
Daleau, P. 1999. Lysophosphatidylcholine, a metabolite which accumulates early in myocardium during ischemia, reduces gap junctional coupling in cardiac cells. Journal of Molecular and Cellular Cardiology 31: 1391–1401.
Fuchs, B., J. Schiller, U. Wagner, H. Häntzschel, and K. Arnold. 2005. The phosphatidylcholine /lysophosphatidylcholine ratio in human plasma is an indicator of the severity of rheumatoid arthritis: Investigations by 31P NMR and MALDI-TOF MS. Clinical Biochemistry 38: 925–933.
Muralikrishna Adibhatla, R., and J.F. Hatcher. 2006. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radical Biology & Medicine 40: 376–387.
Shi, Y., P. Zhang, L. Zhang, H. Osman, E.R. Mohler, C. Macphee, A. Zalewski, A. Postle, and R.L. Wilensky. 2007. Role of lipoprotein-associated phospholipase A2 in leukocyte activation and inflammatory responses. Atherosclerosis 191: 54–62.
Fuentes, L., M. Hernández, F.J. Fernández-Avilés, M.S. Crespo, and M.L. Nieto. 2002. Cooperation between secretory phospholipase A2 and TNF-receptor superfamily signaling: implications for the inflammatory response in atherogenesis. Circulation Research 91: 681–688.
Colles, S.M., and G.M. Chisolm. 2000. Lysophosphatidylcholine-induced cellular injury in cultured fibroblasts involves oxidative events. Journal of Lipid Research 41: 1188–1198.
Matsubara, M., and K. Hasegawa. 2005. Benidipine, a dihydropyridine-calcium channel blocker, prevents lysophosphatidylcholine-induced injury and reactive oxygen species production in human aortic endothelial cells. Atherosclerosis 178: 57–66.
Takeshita, S., N. Inoue, D. Gao, Y. Rikitake, S. Kawashima, R. Tawa, H. Sakurai, and M. Yokoyama. 2000. Lysophosphatidylcholine enhances superoxide anions production via endothelial NADH/NADPH oxidase. Journal of Atherosclerosis and Thrombosis 7: 238–246.
Silliman, C.C., D.J. Elzi, D.R. Ambruso, R.J. Musters, C. Hamiel, R.J. Harbeck, A.J. Paterson, A.J. Bjornsen, T.H. Wyman, M. Kelher, K.M. England, N. McLaughlin-Malaxecheberria, C.C. Barnett, J. Aiboshi, and A. Bannerjee. 2003. Lysophosphatidylcholines prime the NADPH oxidase and stimulate multiple neutrophil functions through changes in cytosolic calcium. Journal of Leukocyte Biology 73: 511–524.
Park, C.H., M.R. Kim, J.M. Han, T.S. Jeong, and D.E. Sok. 2009. Lysophosphatidylcholine exhibits a selective cytotoxicity, accompanied by ROS formation, in RAW 264.7 macrophages. Lipids 44: 425–435.
Huang, L.S., M.R. Kim, and D.E. Sok. 2008. Regulation of lipoxygenase activity by polyunsaturated lysophosphatidylcholines or their oxygenation derivatives. Journal of Agricultural and Food Chemistry 56: 7808–7814.
Funk, C.D. 2001. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 294: 1871–1875.
Hung, N.D., M.R. Kim, and D.E. Sok. 2009. Anti-inflammatory action of arachidonoyl lysophosphatidylcholine or 15-hydroperoxy derivative in zymosan A-induced peritonitis. Prostaglandins & Other Lipid Mediators 90(3–4): 105–111.
Huang, L.S., N.D. Hung, M.R. Kim, and D.E. Sok. 2010. Lysophosphatidylcholine containing docosahexaenoic acid at the sn-1 position is anti-inflammatory. Lipids 45(3): 225–236.
Subbaiah, P.V., M. Liu, and F. Paltauf. 1994. Role of sn-2 acyl group of phosphatidylcholine in determining the positional specificity of lecithin-cholesterol acyltransferase. Biochemistry 33(45): 13259–13266.
Subbaiah, P.V., and M. Liu. 1996. Comparative studies on substrate specificity of lecithin-cholesterol acyltransferase towards the molecular species of phospholipids in plasma of 14 vertebrates. Journal of Lipid Research 37: 113–122.
Gauster, M., G. Rechberger, A. Sovic, G. Horl, E. Steyrer, W. Sattler, and S. Frank. 2005. Endothelial lipase releases saturated and unsaturated fatty acids of high density lipoprotein phosphatidylcholine. Journal of Lipid Research 46: 1517–1525.
Chen, S., and P.V. Subbaiah. 2007. Phospholipid and fatty acid specificity of endothelial lipase: Potential role of the enzyme in the delivery of docosahexaenoic acid (DHA) to tissues. Biochimica et Biophysica Acta 1771(10): 1319–1328.
Cedars, A., C.M. Jenkins, D.J. Mancuso, and R.W. Gross. 2009. Calcium-independent phospholipases in the heart: Mediators of cellular signaling, bioenergetics, and ischemia-induced electrophysiologic dysfunction. Journal of Cardiovascular Pharmacology 53(4): 277–289.
Satouchi, K., M. Sakaguchi, M. Shirakawa, K. Hirano, and T. Tanaka. 1994. Lysophosphatidylcholine from white muscle of bonito Euthynnus pelamis (Linnaeus): Involvement of phospholipase A1 activity for its production. Biochimica et Biophysica Acta 1214(3): 303–308.
Huang, L.S., M.R. Kim, and D.E. Sok. 2006. Linoleoyl lysophosphatidylcholine is an efficient substrate for soybean lipoxygenase-1. Archives of Biochemistry and Biophysics 455: 119–126.
Huang, L.S., M.R. Kim, and D.E. Sok. 2007. Oxygenation of 1-docosahexaenoyl lysophosphatidylcholine by lipoxygenases; conjugated hydroperoxydiene and dihydroxytriene derivatives. Lipids 42: 981–990.
Huang, L.S., M.R. Kim, and D.E. Sok. 2008. Oxygenation of arachidonoyl lysophospholipids by lipoxygenases from soybean, porcine leukocyte, or rabbit reticulocyte. Journal of Agricultural and Food Chemistry 56: 1224–1232.
Tokumura, A., J. Sinomiya, S. Kishimoto, T. Tanaka, K. Kogure, T. Sugiura, K. Satouchi, K. Waku, and K. Fukuzawa. 2002. Human platelets respond differentially to lysophosphatidic acids having a highly unsaturated fatty acyl group and alkyl ether-linked lysophosphatidic acids. The Biochemical Journal 365(3): 617–628.
Bligh, E.G., and W.J. Dyer. 1959. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology 37: 911–917.
Polette, A., C. Deshayes, B. Chantegrel, M. Croset, J.M. Armstrong, and M. Lagarde. 1999. Synthesis of acetyl, docosahexaenoyl-glycerophosphocholine and its characterization using nuclear magnetic resonance. Lipids 34(12): 1333–1337.
Cho, Y.S., H.S. Kim, C.H. Kim, and H.G. Cheon. 2006. Application of the ferrous oxidation-xylenol orange assay for the screening of 5-lipoxygenase inhibitors. Analytical Biochemistry 351: 62–68.
Saw, C.L., Y. Huang, and A.N. Kong. 2010. Synergistic anti-inflammatory effects of low doses of curcumin in combination with polyunsaturated fatty acids: Docosahexaenoic acid or eicosapentaenoic acid. Biochemical Pharmacology 79(3): 421–430.
Aldridge, C., A. Razzak, T.A. Babcock, W.S. Helton, and N.J. Espat. 2008. Lipopolysaccharide-stimulated RAW 264.7 macrophage inducible nitric oxide synthase and nitric oxide production is decreased by an omega-3 fatty acid lipid emulsion. The Journal of Surgical Research 149(2): 296–302.
Kobori, M., H. Nakayama, K. Fukushima, M. Ohnishi-Kameyama, H. Ono, T. Fukushima, Y. Akimoto, S. Masumoto, C. Yukizaki, Y. Hoshi, T. Deguchi, and M. Yoshida. 2008. Bitter gourd suppresses lipopolysaccharide-induced inflammatory responses. Journal of Agricultural and Food Chemistry 56(11): 4004–4011.
Moon, Y., and J.J. Pestka. 2003. Deoxynivalenol-induced mitogen-activated protein kinase phosphorylation and IL-6 expression in mice suppressed by fish oil. The Journal of Nutritional Biochemistry 14(12): 717–726.
Doherty, N.S., P. Poubelle, P. Borgeat, T.H. Beaver, G.L. Westrich, and N.L. Schrader. 1985. Intraperitoneal injection of zymosan in mice induces pain, inflammation and the synthesis of peptidoleukotrienes and prostaglandin E2. Prostaglandins 30: 769–789.
Rao, T.S., J.L. Currie, A.F. Shaffer, and P.C. Isakson. 1994. In vivo characterization of zymosan-induced mouse peritoneal inflammation. The Journal of Pharmacology and Experimental Therapeutics 269: 917.
Byrum, R.S., J.L. Goulet, J.N. Snouwaert, R.J. Griffiths, and B.H. Koller. 1999. Determination of the contribution of cysteinyl leukotrienes and leukotriene B4 in acute inflammatory responses using 5-lipoxygenase-and leukotriene A4 hydrolase-deficient mice. Journal of Immunology 163: 6810–6819.
Forrest, M.J., P.J. Jose, and T.J. Williams. 1986. Kinetics of the generation and action of chemical mediators in zymosan-induced inflammation of the rabbit peritoneal cavity. British Journal of Pharmacology 89: 719–730.
Kolaczkowska, E., M. Barteczko, B. Plytycz, and B. Arnold. 2008. Role of lymphocytes in the course of murine zymosan-induced peritonitis. Inflammation Research 57(6): 272–278.
Arita, M., F. Bianchini, J. Aliberti, A. Sher, N. Chiang, S. Hong, R. Yang, N.A. Petasis, and C.N. Serhan. 2005. Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. The Journal of Experimental Medicine 201: 713–722.
Sun, Y.P., S.F. Oh, J. Uddin, R. Yang, K. Gotlinger, E. Campbell, Colgan, N.A. Petasis, and C.N. Serhan. 2007. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. The Journal of Biological Chemistry 282(13): 9323–9334.
Bannenberg, G., R.L. Moussignac, K. Gronert, P.R. Devchand, B.A. Schmidt, W.J. Guilford, J.G. Bauman, B. Subramanyam, H.D. Perez, J.F. Parkinson, and C.N. Serhan. 2004. Lipoxins and novel 15-epi-lipoxin analogs display potent anti-inflammatory actions after oral administration. British Journal of Pharmacology 143: 43–52.
Rao, N.L., P.J. Dunford, X. Xue, X. Jiang, K.A. Lundeen, F. Coles, J.P. Riley, K.N. Williams, C.A. Grice, J.P. Edwards, L. Karlsson, and A.M. Fourie. 2007. Anti-inflammatory activity of a potent, selective leukotriene A4 hydrolase inhibitor in comparison with the 5-lipoxygenase inhibitor zileuton. The Journal of Pharmacology and Experimental Therapeutics 321(3): 1154–1160.
Yuhki, K., F. Ushikubi, and H. Naraba. 2008. Prostaglandin I2 plays a key role in zymosan-induced mouse pleurisy. The Journal of Pharmacology and Experimental Therapeutics 325(2): 601–609.
Thiès, F., M.C. Delachambre, M. Bentejac, M. Lagarde, and T. Lecerf. 1992. Unsaturated fatty acids esterified in 2-acyl-1-lysophosphatidylcholine bound to albumin are more efficiently taken up by the young rat brain than the unesterified form. Journal of Neurochemistry 59(3): 1110–1116.
Bernoud, N., L. Fenart, P. Molière, M.P. Dehouck, M. Lagarde, R. Cecchelli, and J. Lecerf. 1999. Preferential transfer of 2-docosahexaenoyl-1-lysophosphatidylcholine through an in vitro blood-brain barrier over unesterified docosahexaenoic acid. Journal of Neurochemistry 72(1): 338–3345.
Hong, S., K. Gronert, P.R. Devchand, R.L. Moussignac, and C.N. Serhan. 2003. Novel docosatrienes and 17 S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. Journal of Biological Chemistry 278(17): 14677–14687.
Guzik, T.J., R. Korbut, and T. Adamek-Guzik. 2003. Nitric oxide and superoxide in inflammation and immune regulation. Journal of Physiology and Pharmacology 54(4): 469–487.
Bloodsworth, A., V.B. O’Donnell, and B.A. Freeman. 2000. Nitric oxide regulation of free radical-and enzyme-mediated lipid and lipoprotein oxidation. Arteriosclerosis, Thrombosis, and Vascular Biology 20(7): 1707–1715.
Triggiani, M., A.N. Fonteh, and F.H. Chilton. 1992. Factors that influence the proportions of platelet-activating factor and 1-acyl-2-acetyl-sn-glycero-3-phosphocholine synthesized by the mast cell. The Biochemical Journal 286(2): 497–503.
Kolaczkowska, E., B. Arnold, and G. Opdenakker. 2008. Gelatinase B/MMP-9 as an inflammatory marker enzyme in mouse zymosan peritonitis”: comparison of phase-specific production by mast cells, macrophages, and neutrophils. Immunobiology 213: 109–124.
Kolaczkowska, E., S. Shahzidi, R. Seljelid, N. Van Rooijen, and B. Plytycz. 2002. Early vascular permeability in murine experimental peritonitis is comediated by residential macrophages and mast cells: Crucial involvement of macrophage-derived cysteinyl-leukotrienes. Inflammation 26: 61–71.
Bazan, N.G. 2008. Neurotrophins induce neuroprotective signaling in the retinal pigment epithelial cell by activating the synthesis of the anti-inflammatory and anti-apoptotic neuroprotectin D1. Advances in Experimental Medicine and Biology 613: 39–44.
Serhan, C.N., R. Yang, K. Martinod, K. Kasuga, P.S. Pillai, T.F. Porter, S.F. Oh, and M. Spite. 2009. Maresins: Novel macrophage mediators with potent antiinflammatory and proresolving actions. The Journal of Experimental Medicine 206: 15–23.
Spite, M., L.V. Norling, L. Summers, R. Yang, D. Cooper, N.A. Petasis, R.J. Flower, M. Perretti, and C.N. Serhan. 2009. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 461(7268): 1287–1291.
Acknowledgement
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF 2009-0069242).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hung, N.D., Kim, M.R. & Sok, DE. Oral Administration of 2-Docosahexaenoyl Lysophosphatidylcholine Displayed Anti-Inflammatory Effects on Zymosan A-Induced Peritonitis. Inflammation 34, 147–160 (2011). https://doi.org/10.1007/s10753-010-9218-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-010-9218-z