Abstract
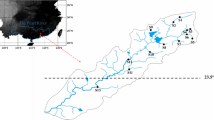
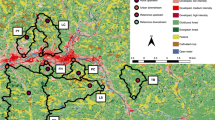
The study of variation of species composition among sites is key to understanding community ecology, but few studies have assessed beta diversity patterns in highly dynamic stream networks in the Neotropical region. We assessed aquatic insect patterns of local contribution to beta diversity (LCBD) and species contribution to beta diversity (SCDB) in a Neotropical drainage network composed of both perennial and intermittent streams in a dry period. We evaluated if environmental and/or spatial predictors drive patterns of LCBD. We sampled aquatic insects in 12 intermittent headwater streams and 34 perennial streams. The intermittent compared to perennial streams had higher LCBDs and lower richness. The pure environmental component significantly explained 19% of the variation of LCBD, while the pure spatial components were not significant. Forty-six taxa contributed to beta diversity above the mean of the 199 taxa. We detected the association of oxygen tolerant and good dispersal ability taxa to intermittent streams and species riffle-adapted taxa as indicators of perennial streams. We showed a disproportional contribution of intermittent streams to the regional species pool. In summary, we demonstrated that when streams dry out, compositional uniqueness may increase during the dry period making them critical to conservation planning of dynamic stream networks.



Similar content being viewed by others
References
Acuña, V., T. Datry, J. Marshall, D. Barcelo, C. N. Dahm, A. Ginebreda, G. McGregor, S. Sabater, K. Tockner & M. A. Palmer, 2014. Why should we care about temporary waterways? Science 343: 1080–1081.
Acuña, V., M. Hunter & A. Ruhí, 2017. Managing temporary streams and rivers as unique rather than second-class ecosystems. Biological Conservation 211: 12–19.
Allan, J. D. & M. M. Castillo, 2007. Stream ecology: structure and function of running waters. Springer, Netherlands.
Altermatt, F., 2013. Diversity in riverine metacommunities: a network perspective. Aquatic Ecology 47: 365–377.
Armstrong, A., R. C. Stedman, J. A. Bishop & P. J. Sullivan, 2012. What’s a stream without water? Disproportionality in headwater regions impacting water quality. Environmental Management 50: 849–860.
Astorga, A., R. Death, F. Death, R. Paavola, M. Chakraborty & T. Muotka, 2014. Habitat heterogeneity drives the geographical distribution of beta diversity: the case of New Zealand stream invertebrates. Ecology and Evolution 4: 2693–2702.
Barbour, M., J. Gerritsen, G. Griffith, R. Frydenborg, E. McCarron, J. White, & M. Bastian, 1996. A framework for biological criteria for Florida streams using benthic macroinvertebrates. Journal of the North American Benthological Society 15: 185–211.
Blanchet, G., P. Legendre & D. Borcard, 2008. Forward selection of spatial explanatory variables. Ecology 89: 2623–2632.
Bogan, M. T. & K. S. Boersma, 2012. Aerial dispersal of aquatic invertebrates along and away from arid-land streams. Freshwater Science 31: 1131–1144.
Borcard, D. & P. Legendre, 2002. All scale spatial analysis of ecological data by means of principal coordinates of neighbor matrices. Ecological Modeling 153: 51–68.
Boulton, A. J., 2003. Parallels and contrasts in the effects of drought on stream macroinvertebrate assemblages. Freshwater Biology 48: 1173–1185.
Cañedo-Argüelles, M., K. S. Boersma, M. T. Bogan, J. D. Olden, I. Phillipsen, T. A. Schriever & D. A. Lytle, 2015. Dispersal strength determines meta-community structure in a dendritic riverine network. Journal of Biogeography 42: 778–790.
Casas, J. J. & M. O. Gessner, 1999. Leaf litter breakdown in a Mediterranean stream characterised by travertine precipitation. Freshwater Biology 41: 781–793.
Clements, W. H. & C. Kotalik, 2016. Effects of major ions on natural benthic communities: an experimental assessment of the US Environmental Protection Agency aquatic life benchmark for conductivity. Freshwater Science 35: 126–138.
Datry, T., 2012. Benthic and hyporheic invertebrate assemblages along a flow intermittence gradient: effects of duration of dry events. Freshwater Biology 57: 563–574.
Datry, T., R. Corti, C. Claret & M. Philippe, 2011. Flow intermittence controls leaf litter breakdown in a French temporary alluvial river: the “drying memory”. Aquatic Sciences 73: 471–483.
Datry, T., S. T. Larned & K. Tockner, 2014. Intermittent rivers: a challenge for freshwater ecology. BioScience 64: 229–235.
Datry, T., N. Bonada & J. Heino, 2016a. Towards understanding the organisation of metacommunities in highly dynamic ecological systems. Oikos 125: 149–159.
Datry, T., A. S. Melo, N. Moya, J. Zubieta, E. De la Barra & T. Oberdorff, 2016b. Metacommunity patterns across three Neotropical catchments with varying environmental harshness. Freshwater Biology 61: 277–292.
Datry, T., N. Bonada & A. Boulton, 2017a. Intermittent Rivers and Ephemeral Streams. Ecology and Management. Academic Press, New York.
Datry, T., R. Corti, J. Heino, B. Hugueny, R. J. Rolls, & A. Ruhí, 2017b. Habitat fragmentation and metapopulation, metacommunity, and metaecosystem dynamics in intermittent rivers and ephemeral streams. Intermittent Rivers and Ephemeral Streams: Ecology and Management. Academic Press
Dormann, C. F., J. Elith, S. Bacher, C. Buchmann, G. Carl, G. Carré, J. R. G. Marquéz, B. Gruber, B. Lafourcade, P. J. Leitão, T. Münkemüller, C. McClean, P. E. Osborne, B. Reineking, B. Schröder, A. K. Skidmore, D. Zurell & S. Lautenbach, 2013. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36: 27–46.
Dray, A. S., G. Blanchet, D. Borcard, S. Clappe, G. Guenard, T. Jombart, G. Larocque, P. Legendre, N. Madi, & H. H. Wagner, 2017. Package ‘adespatial.’
Dray, S., P. Legendre & P. R. Peres-Neto, 2006. Spatial modelling: a comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). Ecological Modelling 196: 483–493.
Dray, S., P. Legendre, & G. Blanchet, 2011. Packfor: forward selection with permutation.
Dufrêne, M. & P. Legendre, 1997. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monographs 67: 345–366.
Fisher, S. G., L. J. Gray, N. B. Grimm & D. E. Busch, 1982. Temporal succession in a desert stream ecosystem following flash flooding. Ecological Monographs 52: 93–110.
Flecker, A. S. & B. Feifarek, 1994. Disturbance and the temporal variability of invertebrate assemblages in two Andean streams. Freshwater Biology 31: 131–142.
Griffith, M. B., 2014. Natural variation and current reference for specific conductivity and major ions in wadeable streams of the conterminous USA. Freshwater Science 33: 1–17.
Griffith, D. A. & P. R. Peres-Neto, 2006. Spatial modeling in ecology: the flexibility of eigenfunction spatial analyses. Ecology 87: 2603–2613.
Heino, J. & M. Grönroos, 2017. Exploring species and site contributions to beta diversity in stream insect assemblages. Oecologia 183: 151–160.
Heino, J., A. S. Melo, L. M. Bini, F. Altermatt, S. A. Al-Shami, D. G. Angeler, N. Bonada, C. Brand, M. Callisto, K. Cottenie, O. Dangles, D. Dudgeon, A. Encalada, E. Göthe, M. Grönroos, N. Hamada, D. Jacobsen, V. L. Landeiro, R. Ligeiro, R. T. Martins, M. L. Miserendino, C. S. Md Rawi, M. E. Rodrigues, F. O. de Roque, L. Sandin, D. Schmera, L. F. Sgarbi, J. P. Simaika, T. Siqueira, R. M. Thompson & C. R. Townsend, 2015. A comparative analysis reveals weak relationships between ecological factors and beta diversity of stream insect metacommunities at two spatial levels. Ecology and Evolution 5: 1235–1248.
IMASUL, 2014. Sistema de Suporte ao Licenciamento Ambiental., http://sisla.imasul.ms.gov.br/sisla.
INMET, 2018. Instituto Nacional de Metereologia - National Institute of Meteorology. Data available at: http://www.inmet.gov.br/portal/index.php?r=home/page&page=rede_estacoes_auto_graf.
Landeiro, V. L., B. Franz, J. Heino, T. Siqueira & L. M. Bini, 2018. Species-poor and low-lying sites are more ecologically unique in a hyperdiverse Amazon region: evidence from multiple taxonomic groups. Diversity and Distributions 24: 966–977.
Legendre, P. & M. De Cáceres, 2013. Beta diversity as the variance of community data: dissimilarity coefficients and partitioning. Ecology Letters 16: 951–963.
Legendre, P., M. R. T. Dale, M.-J. Fortin, J. Gurevitch, M. Hohn & D. Myers, 2002. The consequences of spatial structure for the design and analysis of ecological field surveys. Ecography 25: 601–615.
Leibold, M. A. & J. M. Chase, 2018. Metacommunity Ecology. Princeton University Press, Princeton.
Leibold, M. A., M. Holyoak, N. Mouquet, P. Amarasekare, J. M. Chase, M. F. Hoopes, R. D. Holt, J. B. Shurin, R. Law, D. Tilman, M. Loreau & A. Gonzalez, 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecology Letters 7: 601–613.
Leigh, C., A. J. Boulton, J. L. Courtwright, K. Fritz, C. L. May, R. H. Walker & T. Datry, 2016. Ecological research and management of intermittent rivers: an historical review and future directions. Freshwater Biology 61: 1181–1199.
Leigh, C., K. S. Boersma, M. L. Galatowitsch, V. S. Milner & R. Stubbington, 2019. Are all rivers equal? The role of education in attitudes towards temporary and perennial rivers. People and Nature. https://doi.org/10.1002/pan3.22.
Martínez, A., J. Pérez, J. Molinero, M. Sagarduy & J. Pozo, 2015. Effects of flow scarcity on leaf-litter processing under oceanic climate conditions in calcareous streams. Science of The Total Environment 503–504: 251–257.
Mills, L. S., M. E. Soule & D. F. Doak, 1993. The keystone-species concept in ecology and conservation. BioScience 43: 219–224.
Mouquet, N., D. Gravel, F. Massol & V. Calcagno, 2013. Extending the concept of keystone species to communities and ecosystems. Supporting Information. Ecology Letters 16: 1–8.
Myers, N., R. A. Mittermeier, C. G. Mittermeier, G. A. B. Da Fonseca & J. Kent, 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858.
Oksanen, J., R. Kindt, P. Legendre, B. O’Hara, G. L. Simpson, P. Solymos, H. Stevens, & H. Wagner, 2009. The Vegan Package: Communiity Ecology Package.
Peres-Neto, P. R. & P. Legendre, 2010. Estimating and controlling for spatial structure in the study of ecological communities. Global Ecology and Biogeography 19: 174–184.
Poff, N. L., J. D. Allan, M. B. Bain, J. R. Karr, K. L. Prestegaard, B. D. Richter, R. E. Sparks & J. C. Stromberg, 1997. The natural flow regime. BioScience 47: 769–784.
Roberts, D. W., 2016. Labdsv: Ordination and multivariate analysis in ecology.
Ruhí, A., T. Datry & J. L. Sabo, 2017. Interpreting beta-diversity components over time to conserve metacommunities in highly dynamic ecosystems. Conservation Biology 31: 1459–1468.
Schmera, D., D. Árva, P. Boda, E. Bódis, Á. Bolgovics, G. Borics, A. Csercsa, C. Deák, E. Á. Krasznai, B. A. Lukács, P. Mauchart, A. Móra, P. Sály, A. Specziár, K. Süveges, I. Szivák, P. Takács, M. Tóth, G. Várbíró, A. E. Vojtkó & T. Erős, 2018. Does isolation influence the relative role of environmental and dispersal-related processes in stream networks? An empirical test of the network position hypothesis using multiple taxa. Freshwater Biology 63: 74–85.
Silva, M. B., L. H. C. dos Anjos, M. G. Pereira, J. A. Schiavo, M. Cooper & R. S. de Cavassani, 2017. Soils in the karst landscape of Bodoquena plateau in cerrado region of Brazil. CATENA 154: 107–117.
Socolar, J. B., J. J. Gilroy, W. E. Kunin & D. P. Edwards, 2016. How should beta-diversity inform biodiversity conservation? Trends in Ecology & Evolution 31: 67–80.
Sokal, R. R. & N. L. Oden, 1978. Spatial autocorrelation i n biology 1. Methodology. Biological Journal of the Linnean Society 10: 199–228.
Sor, R., P. Legendre & S. Lek, 2018. Uniqueness of sampling site contributions to the total variance of macroinvertebrate communities in the Lower Mekong Basin. Ecological Indicators 84: 425–432.
Soria, M., C. Leigh, T. Datry, L. M. Bini & N. Bonada, 2017. Biodiversity in perennial and intermittent rivers: a meta-analysis. Oikos 126: 1078–1089.
Strassburg, B. B. N., T. Brooks, R. Feltran-Barbieri, A. Iribarrem, R. Crouzeilles, R. Loyola, A. E. Latawiec, F. J. B. Oliveira Filho, C. A. M. De Scaramuzza, F. R. Scarano, B. Soares-Filho & A. Balmford, 2017. Moment of truth for the Cerrado hotspot. Nature Ecology and Evolution 1: 1–3.
Tolonen, K. E., K. Leinonen, J. Erkinaro & J. Heino, 2018. Ecological uniqueness of macroinvertebrate communities in high-latitude streams is a consequence of deterministic environmental filtering processes. Aquatic Ecology 52: 17–33.
Tomas, W. M., F. de Oliveira Roque, R. G. Morato, P. E. Medici, R. M. Chiaravalloti, F. R. Tortato, J. M. F. Penha, T. J. Izzo, L. C. Garcia, R. F. F. Lourival, P. Girard, N. R. Albuquerque, M. Almeida-Gomes, M. H. da S. Andrade, F. A. S. Araujo, A. C. Araujo, E. C. de Arruda, V. A. Assunção, L. D. Battirola, M. Benites, F. P. Bolzan, J. C. Boock, I. M. Bortolotto, M. da S. Brasil, A. R. Camilo, Z. Campos, M. A. Carniello, A. C. Catella, C. C. Cheida, P. G. Crawshaw, S. M. A. Crispim, G. A. D. Junior, A. L. J. Desbiez, F. A. Dias, D. P. Eaton, G. P. Faggioni, M. A. Farinaccio, J. F. A. Fernandes, V. L. Ferreira, E. A. Fischer, C. E. Fragoso, G. O. Freitas, F. Galvani, A. S. Garcia, C. M. Garcia, G. Graciolli, R. D. Guariento, N. M. R. Guedes, A. Guerra, H. M. Herrera, R. Hoogesteijn, S. C. Ikeda, R. S. Juliano, D. L. Z. K. Kantek, A. Keuroghlian, A. C. R. Lacerda, A. L. R. Lacerda, V. L. Landeiro, R. R. Laps, V. Layme, P. Leimgruber, F. L. Rocha, S. Mamede, D. K. S. Marques, M. I. Marques, L. A. F. Mateus, R. N. Moraes, T. A. Moreira, G. M. Mourão, R. D. Nicola, D. G. Nogueira, A. P. Nunes, C. da Nunes da Cunha, M. D. Oliveira, M. R. Oliveira, G. M. Paggi, A. O. Pellegrin, G. M. F. Pereira, I. A. H. F. S. Peres, J. B. Pinho, J. O. P. Pinto, A. Pott, D. B. Provete, V. D. A. dos Reis, L. K. dos Reis, P.-C. Renaud, D. B. Ribeiro, O. C. Rossetto, J. Sabino, D. Rumiz, S. M. Salis, D. J. Santana, S. A. Santos, Â. L. Sartori, M. Sato, K.-L. Schuchmann, E. Scremin-Dias, G. H. F. Seixas, F. Severo-Neto, M. R. Sigrist, A. Silva, C. J. Silva, A. L. Siqueira, B. M. A. Soriano, L. M. Sousa, F. L. Souza, C. Strussmann, L. S. M. Sugai, N. Tocantins, C. Urbanetz, F. Valente-Neto, D. P. Viana, A. Yanosky, & W. J. Junk, 2019. Sustainability Agenda for the Pantanal Wetland: Perspectives on a Collaborative Interface for Science, Policy, and Decision-Making. Tropical Conservation Science 12: 1–30
Tonkin, J. D., J. Heino, A. Sundermann, P. Haase & S. C. Jähnig, 2016. Context dependency in biodiversity patterns of central German stream metacommunities. Freshwater Biology 61: 607–620.
Tonkin, J. D., F. Altermatt, D. Finn, J. Heino, J. D. Olden, S. U. Pauls, D. A. Lytle, D. S. Finn, J. Heino, J. D. Olden, S. U. Pauls & D. A. Lytle, 2018. The role of dispersal in river network metacommunities: patterns, processes, and pathways. Freshwater Biology 63: 141–163.
Valente-Neto, F., L. Durães, T. Siqueira & F. O. Roque, 2018a. Metacommunity detectives: confronting models based on niche and stochastic assembly scenarios with empirical data from a tropical stream network. Freshwater Biology 63: 86–99.
Valente-Neto, F., M. E. Rodrigues & F. O. de Roque, 2018b. Selecting indicators based on biodiversity surrogacy and environmental response in a riverine network: bringing operationality to biomonitoring. Ecological Indicators 94: 198–206.
Warfe, D. M., N. E. Pettit, R. H. Magierowski, B. J. Pusey, P. M. Davies, M. M. Douglas & S. E. Bunn, 2013. Hydrological connectivity structures concordant plant and animal assemblages according to niche rather than dispersal processes. Freshwater Biology 58: 292–305.
Acknowledgements
We are grateful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT) by grants that allowed the fieldwork. We are also thankful to two anonymous reviewers and to the associate editor Luis Mauricio Bini for their valuable contributions that improved this manuscript. The research was partially supported by the Long Term Ecological Research “Planalto da Bodoquena: redes de interações em longo Prazo” (CNPq-Fundect). Specifically, FVN was supported by grant number 88882.317337/2019-01, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and FOR was supported by CNPq grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Luis Mauricio Bini
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Valente-Neto, F., da Silva, F.H., Covich, A.P. et al. Streams dry and ecological uniqueness rise: environmental selection drives aquatic insect patterns in a stream network prone to intermittence. Hydrobiologia 847, 617–628 (2020). https://doi.org/10.1007/s10750-019-04125-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-04125-9