Abstract
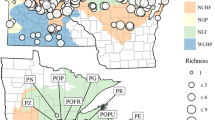
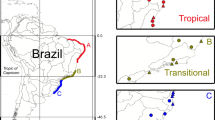
Knowledge of the environmental correlates of species’ distributions is essential for understanding population dynamics, responses to environmental changes, biodiversity patterns, and the impacts of conservation plans. Here we examine how environment controls the distribution of the neotropical genus Montrichardia at regional and local spatial scales using species distribution models (SDMs) and logistic regression, respectively. Montrichardia is a genus of aquatic macrophytes with two species, Montrichardia linifera and Montrichardia arborescens, and is often an important component of flooded habitats. We find that for each species, altitude, precipitation and temperature of the driest month figure in the best performing SDMs as the most important factors controlling large-scale distributions, suggesting that the range limits of both species are climatically constrained by plant water-energy balance and cold intolerance. At small spatial scales, logistic regression models indicate the species partition types of aquatic habitat along local gradients of water pH, conductivity, and water transparency. In summary, a hierarchy of factors may control Montrichardia distribution from large to small spatial scales. While at large spatial scales, evolutionarily conserved climatic niches may control the range limits of the genus, at small spatial scales niche differentiation allows individual species to grow in environmentally distinct aquatic habitats.






Similar content being viewed by others
References
Adams, M. P., M. I. Saunders, P. S. Maxwell, D. Tuazon, C. M. Roelfsema, D. P. Callaghan, J. Leon, R. G. Alistair & K. R. O’Brien, 2015. Prioritizing localized management actions for seagrass conservation and restoration using a species distribution model. Marine and Freshwater Ecosystems, Aquatic Conservation. doi:10.1002/aqc.2573.
Barendregt, A. & A. M. F. Bio, 2003. Relevant variables to predict macrophytes communities in running waters. Ecological Modelling 160: 205–217.
Bornette, G. & S. Puijalon, 2011. Response of aquatic plants to abiotic factors: a review. Aquatic Sciences 73(1): 1–14.
Brown, D. G., 1994. Predicting vegetation types at tree line using topography and biophysical disturbance variables. Journal of Vegetation Science 5: 641–656.
Candolle, A., 1855. Géographie botanique. Paris.
Chambers, P. A., P. Lacoul, K. J. Murphy & S. M. Thomaz, 2008. Global diversity of aquatic macrophytes in freshwater. Hydrobiologia 595: 9–26.
Darwin, C., 1859. The Origin of Species, by Means of Natural Selection. Murray, London.
Dias, R. L., 2009. Softwear Comunidata 1.6.
Efron, B., 1981. Nonparametric estimates of standard error: the jackknife, the bootstrap and other methods. Biometrika 68(3): 589–599.
Elith, J., C. H. Graham, R. P. Anderson, M. Dudik, S. Ferrier, A. Guisan, R. Hijmans, F. R. Huettmann, J. Leathwick, A. Lehmann, J. G. Li, L. A. Lohmann, B. Loiselle, G. Manion, C. Moritz, M. Nakamura, Y. Nakazawa, J. M. M. Overton, A. J. Townsend Peterson, S. Phillips, K. Richardson, R. E. Scachetti-Pereira, R. Schapire, J. Soberón, S. S. Williams, E. Wisz & N. Zimmermann, 2006. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29: 129–151.
Ferreira, F. A. R. P., G. Mormul, A. Pott Catian & G. Pedralli, 2015. Distribution pattern of neotropical aquatic macrophytes in permanent lakes at a Ramsar site. Brazilian Journal of Botany 38(1): 131–139.
Figueroa, J. M. T., M. J. López-Rodríguez, S. Fenoglio, P. Sánchez-Castillo & R. Fochetti, 2013. Freshwater biodiversity in the rivers of the Mediterranean Basin. Hydrobiologia 719(1): 137–186.
Fine, P., D. C. Daly, G. Villa Muñoz, I. Mesones & K. M. Cameron, 2005. The contribution of edaphic heterogeneity to the evolution and diversity of Burseraceae trees in the Western Amazon. Evolution 59(7): 1464–1478.
Freitas, C. T., G. H. Shepard & M. T. F. Piedade, 2015. The floating forest: traditional knowledge and use of matupá vegetation islands by riverine peoples of the Central Amazon. Plos One 10(4): e0122542.
Furch, K., 2000. Chemistry and bioelement inventory of contrasting Amazonian forest soils. In Junk, W. J., J. J. Ohly, M. T. F. Piedade & M. G. M. Soares (eds), The Central Amazon floodplain: actual use and options for a sustainable management. Backhuys Publishers, Leiden: 109–128.
Furch, K. & W. J. Junk, 1997. Physicochemical conditions in the floodplains. Ecological Studies 126: 69–108.
Gentry, A. H., 1988. Changes in plant community diversity and floristic composition on environmental and geographic gradients. Annals of the Missouri Botanical Garden 75(1): 1–34.
Good, R., 1953. The geography of the flowering plants. Longmans Green, London.
Guisan, A. & W. Thuiller, 2005. Predicting species distribution: offering more than simple habitat models. Ecological Letters 8: 993–1009.
Guppy, H., 1906. Observations of a naturalist in the Pacific between 1826 and 1899. II Plant dispersal. Macmillan Publishers Ltd, London.
Householder, E., F. Wittmann, M. Tobler & J. Janovec, 2015. Montane bias in lowland Amazonian peatlands: plant assembly on heterogenous landscapes and potential significance to palynological inference. Palaeogeography, Palaeoclimatology, Palaeoecology 423: 138–148.
IPCC, 2013. Climate Change 2013: the physical science basis. In Stocker, T. F., D. Qin, G.-K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P. M. Midgley (eds), Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK. http://www.climatechange2013.org/images/report/WG1AR5_TS_FINAL.pdf.
Junk, W. J., 1997. The Central Amazon Floodplain: Ecology of a Pulsing System. Ecological Studies, Vol. 126. Springer-Verlag, Berlin: 525.
Junk, W. J. & M. T. F. Piedade, 1997. Plant life in the floodplain with special reference to herbaceous plants. In Junk, W. J. (ed.), The Central Amazon Floodplain, Vol. 126. Springer-Verlag, New York: 147–181.
Junk, W. J., M. T. F. Piedade, J. Schöngart, M. Cohn-Haft, J. M. Adeney & F. Wittmann, 2011. A classification of major naturally-occurring Amazonian lowland wetlands. Wetlands 31: 623–640.
Junk, W. J., M. T. F. Piedade, J. Schöngart & F. Wittmann, 2012. A classification of major natural habitats of Amazonian white water river floodplains (várzeas). Wetlands Ecology and Management 20(6): 461–475.
Junk, W. J., F. Wittmann, J. Schöngart & M. T. F. Piedade, 2015. A classification of the major habitats of Amazonian black-water river floodplains and a comparison with their white-water counterparts. Wetlands Ecology and Management 23(4): 677–693.
Kumar, S. & T. J. Stohlgren, 2009. Maxent modeling for predicting suitable habitat for threatened and endangered tree Canacomyrica monticola in New Caledonia. Journal of Ecology and The Natural Environment 1(4): 094–098.
Lehtonen, S., 2009. On the origin of Echinodorus grandiflorus (Alismataceae) in Florida (“E. floridanus”), and its estimated potential as an invasive species. Hydrobiologia 635(1): 107–112.
Loo, S. E., R. Mac Nally, D. J. O’Dowd, J. R. Thomson & P. S. Lake, 2009. Multiple scale analysis of factors influencing the distribution of an invasive aquatic grass. Biological invasions 11(8): 1903–1912.
Lopes, A. & M. T. F. Piedade, 2014. Experimental study on the survival of the water hyacinth (Eichhornia crassipes (Mart.) Solms-Pontederiaceae) under different oil doses and times of exposure. Environmental Science and Pollution Research 21(23): 13503–13511.
Lopes, A., J. D. A. Paula, S. F. Mardegan, N. Hamada & M. T. F. Piedade, 2011. Influência do hábitat na estrutura da comunidade de macroinvertebrados aquáticos associados às raízes de Eichhornia crassipes na região do Lago Catalão, Amazonas, Brasil. Acta Amazonica 41: 493–502.
Lopes, A., F. Wittmann, J. Schöngart & M. T. F. Piedade, 2014. Herbáceas aquáticas em seis igapós na Amazônia Central: composição e diversidade de gêneros. Revista Geográfica Academica 8(1): 5–17.
Lopes, A., A. B. Ferreira, P. O. Pantoja, P. Parolin & M. T. F. Piedade, 2015. Combined effect of elevated CO2 level and temperature on germination and initial growth of Montrichardia arborescens (L.) Schott (Araceae): a microcosm experiment. Hydrobiologia. doi:10.1007/s10750-015-2598-1.
Lopes, A., P. Parolin & M. T. F. Piedade, 2016. Morphological and physiological traits of aquatic macrophytes respond to water chemistry in the Amazon Basin: an example of the genus Montrichardia Crueg (Araceae). Hydrobiologia 766(1): 1–15.
Mayo, S. J., J. Bogner & P. C. Boyce, 1997. The Genera of Araceae. RBGKew Press, London.
Meave, J., M. Kellman, A. MacDougall & J. Rosales, 1991. Riparian habitats as tropical forest refugia. Global Ecology and Biogeography Letters 1: 69–76.
Merow, C., A. M. Latimer, A. M. Wilson, S. M. McMahon, A. G. Rebelo & J. A. Silander, 2014. On using integral projection models to generate demographically driven predictions of species’ distributions: development and validation using sparse data. Ecography 37(12): 1167–1183.
Neiff, J. J. & A. S. G. Poi de Neiff, 2003. Connectivity processes as a basis for the management of aquatic plants. In Thomaz, S. & L. M. Bini (eds), Ecologia e Manejo de Macrófitas Aquáticas. Nupélia – Maringá. Eduem, Maringá: 39–58.
Pearson, R. G., C. J. Raxworthy, M. Nakamura & A. T. Peterson, 2007. Predicting species’ distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. Journal of Biogeography 34: 102–117.
Phillips, S. J., R. P. Anderson & R. E. Schapire, 2006. Maximum entropy modeling of species geographic distributions. Ecological Modelling 190: 231–259.
Piedade, M. T. F., W. J. Junk, S. A. D’Ângelo, F. Wittmann, J. Schöngart, K. M. D. N. Barbosa & A. Lopes, 2010. Aquatic herbaceous plants of the Amazon floodplains: state of the art and research needed. Acta Limnologica Brasiliensia 22(2): 165–178.
Richey, J. E. E., J. I. Hedges, A. H. Devol, P. D. Quay, R. Victoria, L. Martinelli & B. R. Forsberg, 1990. Biogeochemistry of carbon in the Amazon River. Limnol. Oceanogr 35(2): 352–371.
Santamaría, L., 2002. Why are most aquatic plants widely distributed? Dispersal, clonal growth and small-scale heterogeneity in a stressful environment. Acta Oecologica 23(3): 137–154.
Schöngart, J., M. T. F. Piedade, F. Wittmann, W. J. Junk & M. Worbes, 2005. Wood growth patterns of Macrolobium acaciifolium (Benth.) Benth. (Fabaceae) in Amazonian black-water and white-water floodplain forests. Oecologia 145: 454–461.
Sculthorpe, C. D., 1985. The Biology of Aquatic Vascular Plants. Edward Arnold, London: 610.
Short, F. T. & H. A. Neckles, 1999. The effects of global climate change on seagrasses. Aquatic Botany 63(3): 169–196.
Silvertown, J., M. Dodd, D. Gowing & O. Mountford, 1999. Hydrologically defined niches reveal a basis for species richness in plant communities. Nature 400: 61–63.
Sioli, H., 1968. Hydrochemistry and geology in the Brazilian Amazon region. Amazoniana 3: 267–277.
Weddell, H., 1872. Sur les Podostémacées en général, et leur distribution géographique en particulier. Bulletin de la Société botanique de France 19: 50–57.
Wiens, J. J., 2011. The niche, biogeography and species interactions. Philosophical Transactions of the Royal Society of London B: Biological Sciences 366(1576): 2336–2350.
Wittmann, F., W. J. Junk & M. T. Piedade, 2004. The várzea forests in Amazonia: flooding and the highly dynamic geomorphology interact with natural forest succession. Forest Ecology and Management 196: 199–212.
Acknowledgments
This work is support by INCT ADAPTA—Brazilian Ministry of Science, Technology and Innovation (Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq/Fundação de Amparo à Pesquisa do Estado do Amazonas—FAPEAM), and the Universal CNPq (14/2009; 14/2011), PRONEX “Áreas alagáveis” (CNPq/FAPEAM), PELD MAUA (CNPq/FAPEAM) and FAPEAM EDITAL N. 017/2014—FIXAM/AM Nº Processo: 062.01174/2015 to Dr. Aline Lopes. We thank Marcelo Santos Junior and Marina Anciães for their help with MAXENT Software, and Conceição Lucia Costa, Celso R. Costa, and Valdeney de A. Azevedo for their efforts in collecting field data. Aline Lopes thanks CNPq for her Doctoral Grant and the MAUA Research Group at INPA for logistical and technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Adalberto L. Val, Gudrun De Boeck & Sidinei M. Thomaz / Adaptation of Aquatic Biota of the Amazon
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lopes, A., Wittmann, F., Schöngart, J. et al. Modeling of regional- and local-scale distribution of the genus Montrichardia Crueg. (Araceae). Hydrobiologia 789, 45–57 (2017). https://doi.org/10.1007/s10750-016-2721-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2721-y