Abstract
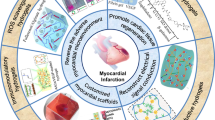
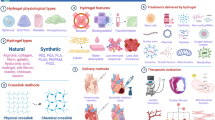
Cardiovascular diseases (CVDs) pose a serious threat to human health, which are characterized by high disability and mortality rate globally such as myocardial infarction (MI), atherosclerosis, and heart failure. Although stem cells transplantation and growth factors therapy are promising, their low survival rate and loss at the site of injury are major obstacles to this therapy. Recently, the development of hydrogel scaffold materials provides a new way to solve this problem, which have shown the potential to treat CVD. Among these scaffold materials, environmentally responsive hydrogels have great prospects in repairing the microenvironment of cardiovascular tissues and vascular regeneration. They provide a new method for the treatment of cardiovascular tissue repair and space-time control for the release of various therapeutic drugs, including small-molecule drugs, growth factors, and stem cells. Herein, this article reviews the occurrence and current treatment of CVD, as well as the repair of cardiovascular injury by several environmental responsive hydrogels systems currently used, mainly focusing on the delivery of growth factors or the application of cell therapy to revascularization. In addition, we will also discuss the enormous potential and personal perspectives of environmentally responsive hydrogels in cardiovascular repair.


Similar content being viewed by others
References
Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, Cipolla CM (2016) Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J Clin 66(4):309–325. https://doi.org/10.3322/caac.21341
Koopman JJE, Kuipers RS (2017) From arterial ageing to cardiovascular disease. Lancet 389(10080):1676–1678. https://doi.org/10.1016/S0140-6736(17)30763-8
Zhu Y, Swanson KM, Rojas RL, Wang Z, St Sauver JL, Visscher SL, Prokop LJ, Bielinski SJ, Wang L, Weinshilboum R, Borah BJ (2019) Systematic review of the evidence on the cost-effectiveness of pharmacogenomics-guided treatment for cardiovascular diseases. Genet Med. https://doi.org/10.1038/s41436-019-0667-y
Collaborators GBDCoD (2017) Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390(10100):1151–1210. https://doi.org/10.1016/S0140-6736(17)32152-9
Ogle BM, Bursac N, Domian I, Huang NF, Menasche P, Murry CE, Pruitt B, Radisic M, Wu JC, Wu SM, Zhang J, Zimmermann WH, Vunjak-Novakovic G (2016) Distilling complexity to advance cardiac tissue engineering. Sci Transl Med 8(342):342ps313. https://doi.org/10.1126/scitranslmed.aad2304
Sepantafar M, Maheronnaghsh R, Mohammadi H, Rajabi-Zeleti S, Annabi N, Aghdami N, Baharvand H (2016) Stem cells and injectable hydrogels: synergistic therapeutics in myocardial repair. Biotechnol Adv 34(4):362–379. https://doi.org/10.1016/j.biotechadv.2016.03.003
Hasan A, Khattab A, Islam MA, Hweij KA, Zeitouny J, Waters R, Sayegh M, Hossain MM, Paul A (2015) Injectable hydrogels for cardiac tissue repair after myocardial infarction. Adv Sci (Weinh) 2(11):1500122. https://doi.org/10.1002/advs.201500122
Gal D, Thijs B, Glanzel W, Sipido KR (2019) Hot topics and trends in cardiovascular research. Eur Heart J 40(28):2363–2374. https://doi.org/10.1093/eurheartj/ehz282
da Silva LP, Kundu SC, Reis RL, Correlo VM (2019) Electric phenomenon: a disregarded tool in tissue engineering and regenerative medicine. Trends Biotechnol. https://doi.org/10.1016/j.tibtech.2019.07.002
Du Y, Guo JL, Wang J, Mikos AG, Zhang S (2019) Hierarchically designed bone scaffolds: from internal cues to external stimuli. Biomaterials 218:119334. https://doi.org/10.1016/j.biomaterials.2019.119334
Lee AY, Mahler N, Best C, Lee YU, Breuer CK (2014) Regenerative implants for cardiovascular tissue engineering. Transl Res 163(4):321–341. https://doi.org/10.1016/j.trsl.2014.01.014
Lee S, Serpooshan V, Tong XM, Venkatraman S, Lee M, Lee J, Chirikian O, Wu JC, Wu SM, Yang F (2017) Contractile force generation by 3D hiPSC-derived cardiac tissues is enhanced by rapid establishment of cellular interconnection in matrix with muscle-mimicking stiffness. Biomaterials 131:111–120. https://doi.org/10.1016/j.biomaterials.2017.03.039
Efraim Y, Sarig H, Anavy NC, Sarig U, de Berardinis E, Chaw SY, Krishnamoorthi M, Kalifa J, Bogireddi H, Duc TV, Kofidis T, Baruch L, Boey FYC, Venkatraman SS, Machluf M (2017) Biohybrid cardiac ECM-based hydrogels improve long term cardiac function post myocardial infarction. Acta Biomater 50:220–233. https://doi.org/10.1016/j.actbio.2016.12.015
Langer R, Vacanti JP (1993) Tissue engineering. Science 260(5110):920–926. https://doi.org/10.1126/science.8493529
Li Y, Xiao Y, Liu C (2017) The horizon of materiobiology: a perspective on material-guided cell behaviors and tissue engineering. Chem Rev 117(5):4376–4421. https://doi.org/10.1021/acs.chemrev.6b00654
Shinoka T, Breuer CK, Tanel RE, Zund G, Miura T, Ma PX, Langer R, Vacanti JP, Mayer JE Jr (1995) Tissue engineering heart valves: valve leaflet replacement study in a lamb model. Ann Thorac Surg 60(6 Suppl):S513–S516. https://doi.org/10.1016/0003-4975(95)00733-4
Chow A, Stuckey DJ, Kidher E, Rocco M, Jabbour RJ, Mansfield CA, Darzi A, Harding SE, Stevens MM, Athanasiou T (2017) Human induced pluripotent stem cell-derived cardiomyocyte encapsulating bioactive hydrogels improve rat heart function post myocardial infarction. Stem Cell Reports 9(5):1415–1422. https://doi.org/10.1016/j.stemcr.2017.09.003
Davoodi P, Lee LY, Xu Q, Sunil V, Sun Y, Soh S, Wang CH (2018) Drug delivery systems for programmed and on-demand release. Adv Drug Deliv Rev 132:104–138. https://doi.org/10.1016/j.addr.2018.07.002
Carlini AS, Gaetani R, Braden RL, Luo C, Christman KL, Gianneschi NC (2019) Enzyme-responsive progelator cyclic peptides for minimally invasive delivery to the heart post-myocardial infarction. Nat Commun 10(1):1735. https://doi.org/10.1038/s41467-019-09587-y
Seliktar D (2012) Designing cell-compatible hydrogels for biomedical applications. Science 336(6085):1124–1128. https://doi.org/10.1126/science.1214804
He X, Aizenberg M, Kuksenok O, Zarzar LD, Shastri A, Balazs AC, Aizenberg J (2012) Synthetic homeostatic materials with chemo-mechano-chemical self-regulation. Nature 487(7406):214–218. https://doi.org/10.1038/nature11223
Kumacheva E (2012) Hydrogels: the catalytic curtsey. Nat Mater 11(8):665–666. https://doi.org/10.1038/nmat3381
Haraguchi Y, Shimizu T, Yamato M, Okano T (2012) Concise review: cell therapy and tissue engineering for cardiovascular disease. Stem Cells Transl Med 1(2):136–141. https://doi.org/10.5966/sctm.2012-0030
Lee E, Kim D, Kim H, Yoon J (2015) Photothermally driven fast responding photo-actuators fabricated with comb-type hydrogels and magnetite nanoparticles. Sci Rep 5:15124. https://doi.org/10.1038/srep15124
Rufaihah AJ, Seliktar D (2016) Hydrogels for therapeutic cardiovascular angiogenesis. Adv Drug Deliv Rev 96:31–39. https://doi.org/10.1016/j.addr.2015.07.003
Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, Alla F, Alvis-Guzman N, Amrock S, Ansari H, Arnlov J, Asayesh H, Atey TM, Avila-Burgos L, Awasthi A, Banerjee A, Barac A, Barnighausen T, Barregard L, Bedi N, Belay Ketema E, Bennett D, Berhe G, Bhutta Z, Bitew S, Carapetis J, Carrero JJ, Malta DC, Castaneda-Orjuela CA, Castillo-Rivas J, Catala-Lopez F, Choi JY, Christensen H, Cirillo M, Cooper L Jr, Criqui M, Cundiff D, Damasceno A, Dandona L, Dandona R, Davletov K, Dharmaratne S, Dorairaj P, Dubey M, Ehrenkranz R, El Sayed ZM, Faraon EJA, Esteghamati A, Farid T, Farvid M, Feigin V, Ding EL, Fowkes G, Gebrehiwot T, Gillum R, Gold A, Gona P, Gupta R, Habtewold TD, Hafezi-Nejad N, Hailu T, Hailu GB, Hankey G, Hassen HY, Abate KH, Havmoeller R, Hay SI, Horino M, Hotez PJ, Jacobsen K, James S, Javanbakht M, Jeemon P, John D, Jonas J, Kalkonde Y, Karimkhani C, Kasaeian A, Khader Y, Khan A, Khang YH, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Kosen S, Krohn KJ, Kumar GA, Kwan GF, Lal DK, Larsson A, Linn S, Lopez A, Lotufo PA, El Razek HMA, Malekzadeh R, Mazidi M, Meier T, Meles KG, Mensah G, Meretoja A, Mezgebe H, Miller T, Mirrakhimov E, Mohammed S, Moran AE, Musa KI, Narula J, Neal B, Ngalesoni F, Nguyen G, Obermeyer CM, Owolabi M, Patton G, Pedro J, Qato D, Qorbani M, Rahimi K, Rai RK, Rawaf S, Ribeiro A, Safiri S, Salomon JA, Santos I, Santric Milicevic M, Sartorius B, Schutte A, Sepanlou S, Shaikh MA, Shin MJ, Shishehbor M, Shore H, Silva DAS, Sobngwi E, Stranges S, Swaminathan S, Tabares-Seisdedos R, Tadele Atnafu N, Tesfay F, Thakur JS, Thrift A, Topor-Madry R, Truelsen T, Tyrovolas S, Ukwaja KN, Uthman O, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Watkins D, Weintraub R, Werdecker A, Westerman R, Wiysonge CS, Wolfe C, Workicho A, Xu G, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Vos T, Naghavi M, Murray C (2017) Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 70(1):1–25. https://doi.org/10.1016/j.jacc.2017.04.052
Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA (2009) Hydrogels in regenerative medicine. Adv Mater 21(32–33):3307–3329. https://doi.org/10.1002/adma.200802106
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics C, Stroke Statistics S (2017) Heart Disease and Stroke Statistics-2017 update: a report from the American Heart Association. Circulation 135(10):e146–e603. https://doi.org/10.1161/CIR.0000000000000485
Libby P, Ridker PM, Hansson GK (2011) Progress and challenges in translating the biology of atherosclerosis. Nature 473(7347):317–325. https://doi.org/10.1038/nature10146
Bagno L, Hatzistergos KE, Balkan W, Hare JM (2018) Mesenchymal stem cell-based therapy for cardiovascular disease: progress and challenges. Mol Ther 26(7):1610–1623. https://doi.org/10.1016/j.ymthe.2018.05.009
Segers VF, Lee RT (2008) Stem-cell therapy for cardiac disease. Nature 451(7181):937–942. https://doi.org/10.1038/nature06800
Lakatta EG, Levy D (2003) Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation 107(1):139–146. https://doi.org/10.1161/01.cir.0000048892.83521.58
North BJ, Sinclair DA (2012) The intersection between aging and cardiovascular disease. Circ Res 110(8):1097–1108. https://doi.org/10.1161/Circresaha.111.246876
Roth GA, Johnson CO, Abate KH, Abd-Allah F, Ahmed M, Alam K, Alam T, Alvis-Guzman N, Ansari H, Arnlov J, Atey TM, Awasthi A, Awoke T, Barac A, Barnighausen T, Bedi N, Bennett D, Bensenor I, Biadgilign S, Castaneda-Orjuela C, Catala-Lopez F, Davletov K, Dharmaratne S, Ding EL, Dubey M, Faraon EJA, Farid T, Farvid MS, Feigin V, Fernandes J, Frostad J, Gebru A, Geleijnse JM, Gona PN, Griswold M, Hailu GB, Hankey GJ, Hassen HY, Havmoeller R, Hay S, Heckbert SR, Irvine CMS, James SL, Jara D, Kasaeian A, Khan AR, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Lal D, Larsson A, Linn S, Lotufo PA, Abd El Razek HM, Mazidi M, Meier T, Mendoza W, Mensah GA, Meretoja A, Mezgebe HB, Mirrakhimov E, Mohammed S, Moran AE, Nguyen G, Nguyen M, Ong KL, Owolabi M, Pletcher M, Pourmalek F, Purcell CA, Qorbani M, Rahman M, Rai RK, Ram U, Reitsma MB, Renzaho AMN, Rios-Blancas MJ, Safiri S, Salomon JA, Sartorius B, Sepanlou SG, Shaikh MA, Silva D, Stranges S, Tabares-Seisdedos R, Atnafu NT, Thakur JS, Topor-Madry R, Truelsen T, Tuzcu EM, Tyrovolas S, Ukwaja KN, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Weintraub R, Wolfe C, Workicho A, Xu GL, Yadgir S, Yano Y, Yip P, Yonemoto N, Younis M, Yu CH, Zaidi Z, Zaki MES, Zipkin B, Afshin A, Gakidou E, Lim SS, Mokdad AH, Naghavi M, Vos T, Murray CJL, Coll GBCD (2018) The burden of cardiovascular diseases among US states, 1990-2016. JAMA Cardiol 3(5):375–389. https://doi.org/10.1001/jamacardio.2018.0385
Hu D, Li L, Li SF, Wu MY, Ge NN, Cui YX, Lian Z, Song JX, Chen H (2019) Lymphatic system identification, pathophysiology and therapy in the cardiovascular diseases. J Mol Cell Cardiol 133:99–111. https://doi.org/10.1016/j.yjmcc.2019.06.002
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu SM, Mackey RH, Magid DJ, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, Comm AHAS, Subcomm SS (2016) Heart Disease and Stroke Statistics-2016 update a report from the American Heart Association. Circulation 133(4):E38–E360. https://doi.org/10.1161/Cir.0000000000000350
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai SF, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, Assoc AH, Subcomm SS (2013) Heart Disease and Stroke Statistics-2013 update a report from the American Heart Association. Circulation 127(1):E6–E245. https://doi.org/10.1161/CIR.0b013e31828124ad
Dalen JE, Alpert JS, Goldberg RJ, Weinstein RS (2014) The epidemic of the 20(th) century: coronary heart disease. Am J Med 127(9):807–812. https://doi.org/10.1016/j.amjmed.2014.04.015
Zhong S, Li L, Shen X, Li Q, Xu W, Wang X, Tao Y, Yin H (2019) An update on lipid oxidation and inflammation in cardiovascular diseases. Free Radic Biol Med. https://doi.org/10.1016/j.freeradbiomed.2019.03.036
Zhao TX, Mallat Z (2019) Targeting the immune system in atherosclerosis: JACC state-of-the-art review. J Am Coll Cardiol 73(13):1691–1706. https://doi.org/10.1016/j.jacc.2018.12.083
Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Liu LS, Investigators IS (2004) Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 364(9438):937–952. https://doi.org/10.1016/S0140-6736(04)17018-9
Messner B, Bernhard D (2014) Smoking and cardiovascular disease mechanisms of endothelial dysfunction and early atherogenesis. Arterioscl Throm Vas 34(3):509–515. https://doi.org/10.1161/Atvbaha.113.300156
Wang CCL, Hess CN, Hiatt WR, Goldfine AB (2016) Clinical update: cardiovascular disease in diabetes mellitus atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus - mechanisms, management, and clinical considerations. Circulation 133(24):2459–2502. https://doi.org/10.1161/Circulationaha.116.022194
Lv J, Yu CQ, Guo Y, Bian Z, Yang L, Chen YP, Tang XF, Zhang WY, Qian YJ, Huang YL, Wang XP, Chen JS, Chen ZM, Qi L, Li LM, Biobank CK (2017) Adherence to healthy lifestyle and cardiovascular diseases in the Chinese population. J Am Coll Cardiol 69(9):1116–1125. https://doi.org/10.1016/j.jacc.2016.11.076
Behfar A, Crespo-Diaz R, Terzic A, Gersh BJ (2014) Cell therapy for cardiac repair-lessons from clinical trials. Nat Rev Cardiol 11(4):232–246. https://doi.org/10.1038/nrcardio.2014.9
Otto CM (2019) Informed shared decisions for patients with aortic stenosis. New Engl J Med 380(18):1769–1770. https://doi.org/10.1056/NEJMe1903316
Vandvik PO, Otto CM, Siemieniuk RA, Bagur R, Guyatt GH, Lytvyn L, Whitlock R, Vartdal T, Brieger D, Aertgeerts B, Price S, Foroutan F, Shapiro M, Mertz R, Spencer FA (2016) Transcatheter or surgical aortic valve replacement for patients with severe, symptomatic, aortic stenosis at low to intermediate surgical risk: a clinical practice guideline. BMJ 354:i5085. https://doi.org/10.1136/bmj.i5085
Blau HM, Daley GQ (2019) Stem cells in the treatment of disease. N Engl J Med 380(18):1748–1760. https://doi.org/10.1056/NEJMra1716145
Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S, Group ESCSD (2016) 2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European Society of Cardiology and Other Societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts)developed with the special contribution of the European Association for Cardiovascular Prevention & rehabilitation (EACPR). Eur Heart J 37(29):2315–2381. https://doi.org/10.1093/eurheartj/ehw106
Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF, American College of Cardiology/American Heart Association Task Force on Practice G (2014) 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation 129(25 Suppl 2):S1–S45. https://doi.org/10.1161/01.cir.0000437738.63853.7a
Marquis-Gravel G, Roe MT, Harrington RA, Munoz D, Hernandez AF, Jones WS (2019) Revisiting the role of aspirin for the primary prevention of cardiovascular disease. Circulation 140(13):1115–1124. https://doi.org/10.1161/CIRCULATIONAHA.119.040205
Byrne P, Cullinan J, Smith SM (2019) Statins for primary prevention of cardiovascular disease. BMJ 367:l5674. https://doi.org/10.1136/bmj.l5674
Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, Scott R, Koren MJ, Stein EA, Open-Label Study of Long-Term Evaluation against LDLCI (2015) Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 372(16):1500–1509. https://doi.org/10.1056/NEJMoa1500858
Takeuchi R, Kuruma Y, Sekine H, Dobashi I, Yamato M, Umezu M, Shimizu T, Okano T (2016) In vivo vascularization of cell sheets provided better long-term tissue survival than injection of cell suspension. J Tissue Eng Regen M 10(8):700–710. https://doi.org/10.1002/term.1854
Radisic M, Christman KL (2013) Materials science and tissue engineering: repairing the heart. Mayo Clin Proc 88(8):884–898. https://doi.org/10.1016/j.mayocp.2013.05.003
Lutgens E, Atzler D, Doring Y, Duchene J, Steffens S, Weber C (2019) Immunotherapy for cardiovascular disease. Eur Heart J. https://doi.org/10.1093/eurheartj/ehz283
Lu D, Thum T (2019) RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat Rev Cardiol 16(11):661–674. https://doi.org/10.1038/s41569-019-0218-x
Zhang Y, Murugesan P, Huang K, Cai H (2019) NADPH oxidases and oxidase crosstalk in cardiovascular diseases: novel therapeutic targets. Nat Rev Cardiol. https://doi.org/10.1038/s41569-019-0260-8
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, JJP K, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, PRF R, Troquay RPT, Libby P, Glynn RJ, Group CT (2017) Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl J Med 377(12):1119–1131. https://doi.org/10.1056/NEJMoa1707914
Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC Jr, Svetkey LP, Wadden TA, Yanovski SZ, Kendall KA, Morgan LC, Trisolini MG, Velasco G, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF, American College of Cardiology/American Heart Association Task Force on Practice G (2014) 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation 129(25 Suppl 2):S76–S99. https://doi.org/10.1161/01.cir.0000437740.48606.d1
Look ARG, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Reboussin D, Regensteiner JG, Rickman AD, Ryan DH, Safford M, Wadden TA, Wagenknecht LE, West DS, Williamson DF, Yanovski SZ (2013) Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 369(2):145–154. https://doi.org/10.1056/NEJMoa1212914
Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL (2015) Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 163(7):1585–1595. https://doi.org/10.1016/j.cell.2015.11.055
de Lima PR, Duque AP, Moreira BR, Rodrigues LFJ (2019) Acupuncture for the treatment of cardiovascular diseases: a systematic review. J Acupunct Meridian Stud 12(2):43–51. https://doi.org/10.1016/j.jams.2018.07.005
Buikema JW, Van Der Meer P, Sluijter JP, Domian IJ (2013) Concise review: engineering myocardial tissue: the convergence of stem cells biology and tissue engineering technology. Stem Cells 31(12):2587–2598. https://doi.org/10.1002/stem.1467
Radhakrishnan J, Krishnan UM, Sethuraman S (2014) Hydrogel based injectable scaffolds for cardiac tissue regeneration. Biotechnol Adv 32(2):449–461. https://doi.org/10.1016/j.biotechadv.2013.12.010
Ungerleider JL, Christman KL (2014) Concise review: injectable biomaterials for the treatment of myocardial infarction and peripheral artery disease: translational challenges and progress. Stem Cells Transl Med 3(9):1090–1099. https://doi.org/10.5966/sctm.2014-0049
Badeau BA, Comerford MP, Arakawa CK, Shadish JA, DeForest CA (2018) Engineered modular biomaterial logic gates for environmentally triggered therapeutic delivery. Nat Chem 10(3):251–258. https://doi.org/10.1038/nchem.2917
Flegeau K, Pace R, Gautier H, Rethore G, Guicheux J, Le Visage C, Weiss P (2017) Toward the development of biomimetic injectable and macroporous biohydrogels for regenerative medicine. Adv Colloid Interf Sci 247:589–609. https://doi.org/10.1016/j.cis.2017.07.012
Bastings MM, Koudstaal S, Kieltyka RE, Nakano Y, Pape AC, Feyen DA, van Slochteren FJ, Doevendans PA, Sluijter JP, Meijer EW, Chamuleau SA, Dankers PY (2014) A fast pH-switchable and self-healing supramolecular hydrogel carrier for guided, local catheter injection in the infarcted myocardium. Adv Healthc Mater 3(1):70–78. https://doi.org/10.1002/adhm.201300076
Garbern JC, Minami E, Stayton PS, Murry CE (2011) Delivery of basic fibroblast growth factor with a pH-responsive, injectable hydrogel to improve angiogenesis in infarcted myocardium. Biomaterials 32(9):2407–2416. https://doi.org/10.1016/j.biomaterials.2010.11.075
Wang T, Zheng Y, Shi Y, Zhao L (2019) pH-responsive calcium alginate hydrogel laden with protamine nanoparticles and hyaluronan oligosaccharide promotes diabetic wound healing by enhancing angiogenesis and antibacterial activity. Drug Deliv Transl Res 9(1):227–239. https://doi.org/10.1007/s13346-018-00609-8
Wang Q, Xie XL, Zhang XW, Zhang JP, Wang AQ (2010) Preparation and swelling properties of pH-sensitive composite hydrogel beads based on chitosan-g-poly (acrylic acid)/vermiculite and sodium alginate for diclofenac controlled release. Int J Biol Macromol 46(3):356–362. https://doi.org/10.1016/j.ijbiomac.2010.01.009
Wang HB, Zhang XL, Li YM, Ma YT, Zhang Y, Liu ZG, Zhou J, Lin QX, Wang YM, Duan CM, Wang CY (2010) Improved myocardial performance in infarcted rat heart by co-injection of basic fibroblast growth factor with temperature-responsive chitosan hydrogel. J Heart Lung Transplant 29(8):881–887. https://doi.org/10.1016/j.healun.2010.03.016
Lu SH, Wang HB, Lu WN, Liu S, Lin QX, Li DX, Duan C, Hao T, Zhou J, Wang YM, Gao SR, Wang CY (2010) Both the transplantation of somatic cell nuclear transfer- and fertilization-derived mouse embryonic stem cells with temperature-responsive chitosan hydrogel improve myocardial performance in infarcted rat hearts. Tissue Eng Pt A 16(4):1303–1315. https://doi.org/10.1089/ten.tea.2009.0434
Chen CH, Chen SH, Mao SH, Tsai MJ, Chou PY, Liao CH, Chen JP (2017) Injectable thermosensitive hydrogel containing hyaluronic acid and chitosan as a barrier for prevention of postoperative peritoneal adhesion. Carbohydr Polym 173:721–731. https://doi.org/10.1016/j.carbpol.2017.06.019
He D, Zhao AS, Su H, Zhang Y, Wang YN, Luo D, Gao Y, Li JA, Yang P (2019) An injectable scaffold based on temperature-responsive hydrogel and factor-loaded nanoparticles for application in vascularization in tissue engineering. J Biomed Mater Res A 107(10):2123–2134. https://doi.org/10.1002/jbm.a.36723
Mou CL, Ju XJ, Zhang L, Xie R, Wang W, Deng NN, Wei J, Chen QM, Chu LY (2014) Monodisperse and fast-responsive poly(N-isopropylacrylamide) microgels with open-celled porous structure. Langmuir 30(5):1455–1464. https://doi.org/10.1021/la4046379
Kim SJ, Yooon SG, Lee YM, Kim HC, Kim SI (2004) Electrical behavior of polymer hydrogel composed of poly(vinyl alcohol)-hyaluronic acid in solution. Biosens Bioelectron 19(6):531–536. https://doi.org/10.1016/S0956-5663(03)00277-X
O'Neill HS, Herron CC, Hastings CL, Deckers R, Noriega AL, Kelly HM, Hennink WE, McDonnell CO, O'Brien FJ, Ruiz-Hernandez E, Duffy GP (2017) A stimuli responsive liposome loaded hydrogel provides flexible on-demand release of therapeutic agents. Acta Biomater 48:110–119. https://doi.org/10.1016/j.actbio.2016.10.001
Mura S, Nicolas J, Couvreur P (2013) Stimuli-responsive nanocarriers for drug delivery. Nat Mater 12(11):991–1003. https://doi.org/10.1038/nmat3776
Knipe JM, Peppas NA (2014) Multi-responsive hydrogels for drug delivery and tissue engineering applications. Regen Biomater 1(1):57–65. https://doi.org/10.1093/rb/rbu006
Ma C, Li T, Zhao Q, Yang X, Wu J, Luo Y, Xie T (2014) Supramolecular Lego assembly towards three-dimensional multi-responsive hydrogels. Adv Mater 26(32):5665–5669. https://doi.org/10.1002/adma.201402026
Liu Z, Liu L, Ju XJ, Xie R, Zhang B, Chu LY (2011) K(+)-recognition capsules with squirting release mechanisms. Chem Commun (Camb) 47(45):12283–12285. https://doi.org/10.1039/c1cc15082k
Tu T, Fang W, Sun Z (2013) Visual-size molecular recognition based on gels. Adv Mater 25(37):5304–5313. https://doi.org/10.1002/adma.201301914
Yang JM, Olanrele OS, Zhang X, Hsu CC (2018) Fabrication of hydrogel materials for biomedical applications. Adv Exp Med Biol 1077:197–224. https://doi.org/10.1007/978-981-13-0947-2_12
Yeh PD, Alexeev A (2015) Mesoscale modelling of environmentally responsive hydrogels: emerging applications. Chem Commun (Camb) 51(50):10083–10095. https://doi.org/10.1039/c5cc01027f
Gerecht-Nir S, Radisic M, Park H, Cannizzaro C, Boublik J, Langer R, Vunjak-Novakovic G (2006) Biophysical regulation during cardiac development and application to tissue engineering. Int J Dev Biol 50(2–3):233–243. https://doi.org/10.1387/ijdb.052041sg
LeGrice IJ, Smaill BH, Chai LZ, Edgar SG, Gavin JB, Hunter PJ (1995) Laminar structure of the heart: ventricular myocyte arrangement and connective tissue architecture in the dog. Am J Phys 269(2 Pt 2):H571–H582. https://doi.org/10.1152/ajpheart.1995.269.2.H571
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414(6865):813–820. https://doi.org/10.1038/414813a
Li J, Zhang K, Huang N (2017) Engineering cardiovascular implant surfaces to create a vascular endothelial growth microenvironment. Biotechnol J 12(12):1600401. https://doi.org/10.1002/biot.201600401
Longchamp A, Mirabella T, Arduini A, MacArthur MR, Das A, Trevino-Villarreal JH, Hine C, Ben-Sahra I, Knudsen NH, Brace LE, Reynolds J, Mejia P, Tao M, Sharma G, Wang R, Corpataux JM, Haefliger JA, Ahn KH, Lee CH, Manning BD, Sinclair DA, Chen CS, Ozaki CK, Mitchell JR (2018) Amino acid restriction triggers angiogenesis via GCN2/ATF4 regulation of VEGF and H2S production. Cell 173(1):117–129. https://doi.org/10.1016/j.cell.2018.03.001
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473(7347):298–307. https://doi.org/10.1038/nature10144
Bikfalvi A (2017) History and conceptual developments in vascular biology and angiogenesis research: a personal view. Angiogenesis 20(4):463–478. https://doi.org/10.1007/s10456-017-9569-2
Carmeliet P, Jain RK (2011) Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov 10(6):417–427. https://doi.org/10.1038/nrd3455
Viallard C, Larrivee B (2017) Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis 20(4):409–426. https://doi.org/10.1007/s10456-017-9562-9
de Palma M, Biziato D, Petrova TV (2017) Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer 17(8):457–474. https://doi.org/10.1038/nrc.2017.51
Forget A, Gianni-Barrera R, Uccelli A, Sarem M, Kohler E, Fogli B, Muraro MG, Bichet S, Aumann K, Banfi A, Shastri VP (2019) Mechanically defined microenvironment promotes stabilization of microvasculature, which correlates with the enrichment of a novel Piezo-1(+) population of circulating CD11b(+)/CD115(+) monocytes. Adv Mater 31(21):ARTN 1808050. https://doi.org/10.1002/adma.201808050
Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S (2001) Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 7(4):430–436. https://doi.org/10.1038/86498
Yla-Herttuala S, Rissanen TT, Vajanto I, Hartikainen J (2007) Vascular endothelial growth factors - biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol 49(10):1015–1026. https://doi.org/10.1016/j.jacc.2006.09.053
Samanta D, Hosseini-Nassab N, Zare RN (2016) Electroresponsive nanoparticles for drug delivery on demand. Nanoscale 8(17):9310–9317. https://doi.org/10.1039/c6nr01884j
Guo BL, Yuan JF, Gao QY (2008) Preparation and release behavior of temperature- and pH-responsive chitosan material. Polym Int 57(3):463–468. https://doi.org/10.1002/pi.2350
Shao ZQ, Takaji K, Katayama Y, Kunitomo R, Sakaguchi H, Lai ZF, Kawasuji M (2006) Effects of intramyocardial administration of slow-release basic fibroblast growth factor on angiogenesis and ventricular remodeling in a rat infarct model. Circ J 70(4):471–477. https://doi.org/10.1253/circj.70.471
Zhang W, Jin X, Li H, Zhang RR, Wu CW (2018) Injectable and body temperature sensitive hydrogels based on chitosan and hyaluronic acid for pH sensitive drug release. Carbohydr Polym 186:82–90. https://doi.org/10.1016/j.carbpol.2018.01.008
Cheng NC, Lin WJ, Ling TY, Young TH (2017) Sustained release of adipose-derived stem cells by thermosensitive chitosan/gelatin hydrogel for therapeutic angiogenesis. Acta Biomater 51:258–267. https://doi.org/10.1016/j.actbio.2017.01.060
Ron ES, Bromberg LE (1998) Temperature-responsive gels and thermogelling polymer matrices for protein and peptide delivery. Adv Drug Deliv Rev 31(3):197–221
He D, Zhao A-s, Hong S, Zhang Y, Wang Y-n, Luo D, Gao Y, Li J-a, Yang P (2019) An injectable scaffold based on temperature responsive hydrogel and factors loaded nano-particles for potential application of vascularization in tissue engineering. J Biomed Mater Res A 107A:2123–2134
Osman A, Oner ET, Eroglu MS (2017) Novel Levan and pNIPA temperature sensitive hydrogels for 5-ASA controlled release. Carbohydr Polym 165:61–70. https://doi.org/10.1016/j.carbpol.2017.01.097
Hoare T, Santamaria J, Goya GF, Irusta S, Lin D, Lau S, Padera R, Langer R, Kohane DS (2009) A magnetically triggered composite membrane for on-demand drug delivery. Nano Lett 9(10):3651–3657. https://doi.org/10.1021/nl9018935
Liang Z, Liu C, Li L, Xu P, Luo G, Ding M, Liang Q (2016) Double-network hydrogel with tunable mechanical performance and biocompatibility for the fabrication of stem cells-encapsulated fibers and 3D assemble. Sci Rep 6:33462. https://doi.org/10.1038/srep33462
Zhang XZ, Xu XD, Cheng SX, Zhuo RX (2008) Strategies to improve the response rate of thermosensitive PNIPAAm hydrogels. Soft Matter 4(3):385–391. https://doi.org/10.1039/b713803m
Han D, Lu ZC, Chester SA, Lee H (2018) Micro 3D printing of a temperature-responsive hydrogel using projection micro-stereolithography. Sci Rep-Uk 8:ARTN 1963. https://doi.org/10.1038/s41598-018-20385-2
Li X, Zhou J, Liu ZQ, Chen J, Lu SH, Sun HY, Li JJ, Lin QX, Yang BG, Duan CM, Xing M, Wang CY (2014) A PNIPAAm-based thermosensitive hydrogel containing SWCNTs for stem cell transplantation in myocardial repair. Biomaterials 35(22):5679–5688. https://doi.org/10.1016/j.biomaterials.2014.03.067
Zhang K, Shi ZQ, Zhou JK, Xing Q, Ma SS, Li QH, Zhang YT, Yao MH, Wang XF, Li Q, Li JA, Guan FX (2018) Potential application of an injectable hydrogel scaffold loaded with mesenchymal stem cells for treating traumatic brain injury. J Mater Chem B 6(19):2982–2992. https://doi.org/10.1039/c7tb03213g
Karam JP, Muscari C, Sindji L, Bastiat G, Bonafe F, Venier-Julienne MC, Montero-Menei NC (2014) Pharmacologically active microcarriers associated with thermosensitive hydrogel as a growth factor releasing biomimetic 3D scaffold for cardiac tissue-engineering. J Control Release 192:82–94. https://doi.org/10.1016/j.jconrel.2014.06.052
Overstreet DJ, Badha VS, Heffernan JM, Childers EP, Moore RC, Vernon BL, McLaren AC (2019) Temperature-responsive PNDJ hydrogels provide high and sustained antimicrobial concentrations in surgical sites. Drug Deliv Transl Re 9(4):802–815. https://doi.org/10.1007/s13346-019-00630-5
Koetting MC, Peters JT, Steichen SD, Peppas NA (2015) Stimulus-responsive hydrogels: theory, modern advances, and applications. Mater Sci Eng R Rep 93:1–49. https://doi.org/10.1016/j.mser.2015.04.001
Lang WR (1955) Vaginal acidity and pH; a review. Obstet Gynecol Surv 10(4):546–560. https://doi.org/10.1097/00006254-195508000-00009
Wike-Hooley JL, van den Berg AP, van der Zee J, Reinhold HS (1985) Human tumour pH and its variation. Eur J Cancer Clin Oncol 21(7):785–791. https://doi.org/10.1016/0277-5379(85)90216-0
Khabbaz KR, Zankoul F, Warner KG (2001) Intraoperative metabolic monitoring of the heart: II. Online measurement of myocardial tissue pH. Ann Thorac Surg 72(6):S2227–S2233; discussion S2233-2224, S2267-2270. https://doi.org/10.1016/s0003-4975(01)03284-2
Kumbhani DJ, Healey NA, Birjiniuk V, Crittenden MD, Josa M, Treanor PR, Khuri SF (2004) Determinants of regional myocardial acidosis during cardiac surgery. Surgery 136(2):190–198. https://doi.org/10.1016/j.surg.2004.04.015
Matsusaki M, Akashi M (2005) Novel functional biodegradable polymer IV: pH-sensitive controlled release of fibroblast growth factor-2 from a poly(gamma-glutamic acid)-sulfonate matrix for tissue engineering. Biomacromolecules 6(6):3351–3356. https://doi.org/10.1021/bm050369m
Werzer O, Tumphart S, Keimel R, Christian P, Coclite AM (2019) Drug release from thin films encapsulated by a temperature-responsive hydrogel. Soft Matter 15(8):1853–1859. https://doi.org/10.1039/c8sm02529k
Teixeira LS, Feijen J, van Blitterswijk CA, Dijkstra PJ, Karperien M (2012) Enzyme-catalyzed crosslinkable hydrogels: emerging strategies for tissue engineering. Biomaterials 33(5):1281–1290. https://doi.org/10.1016/j.biomaterials.2011.10.067
Tran NQ, Joung YK, Lih E, Park KM, Park KD (2010) Supramolecular hydrogels exhibiting fast in situ gel forming and adjustable degradation properties. Biomacromolecules 11(3):617–625. https://doi.org/10.1021/bm100047y
Yu SS, Scherer RL, Ortega RA, Bell CS, O'Neil CP, Hubbell JA, Giorgio TD (2011) Enzymatic- and temperature-sensitive controlled release of ultrasmall superparamagnetic iron oxides (USPIOs). J Nanobiotechnology 9:7. https://doi.org/10.1186/1477-3155-9-7
Akiyama N, Yamamoto-Fukuda T, Takahashi H, Koji T (2013) In situ tissue engineering with synthetic self-assembling peptide nanofiber scaffolds, PuraMatrix, for mucosal regeneration in the rat middle-ear. Int J Nanomedicine 8:2629–2640. https://doi.org/10.2147/IJN.S47279
Li X, Chen YY, Wang XM, Gao K, Gao YZ, Cao J, Zhang ZL, Lei J, Jin ZY, Wang YN (2017) Image-guided stem cells with functionalized self-assembling peptide nanofibers for treatment of acute myocardial infarction in a mouse model. Am J Transl Res 9(8):3723–3731
Rad-Malekshahi M, Lempsink L, Amidi M, Hennink WE, Mastrobattista E (2016) Biomedical applications of self-assembling peptides. Bioconjug Chem 27(1):3–18. https://doi.org/10.1021/acs.bioconjchem.5b00487
Koutsopoulos S (2016) Self-assembling peptide nanofiber hydrogels in tissue engineering and regenerative medicine: progress, design guidelines, and applications. J Biomed Mater Res A 104(4):1002–1016. https://doi.org/10.1002/jbm.a.35638
Van Hove AH, Burke K, Antonienko E, Brown E 3rd, Benoit DS (2015) Enzymatically-responsive pro-angiogenic peptide-releasing poly(ethylene glycol) hydrogels promote vascularization in vivo. J Control Release 217:191–201. https://doi.org/10.1016/j.jconrel.2015.09.005
Wilson AN, Salas R, Guiseppi-Elie A (2012) Bioactive hydrogels demonstrate mediated release of a chromophore by chymotrypsin. J Control Release 160(1):41–47. https://doi.org/10.1016/j.jconrel.2012.02.026
Hsu CW, Olabisi RM, Olmsted-Davis EA, Davis AR, West JL (2011) Cathepsin K-sensitive poly(ethylene glycol) hydrogels for degradation in response to bone resorption. J Biomed Mater Res A 98(1):53–62. https://doi.org/10.1002/jbm.a.33076
Ziche M, Morbidelli L (2000) Nitric oxide and angiogenesis. J Neuro-Oncol 50(1–2):139–148. https://doi.org/10.1023/a:1006431309841
Lundberg JO, Gladwin MT, Weitzberg E (2015) Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov 14(9):623–641. https://doi.org/10.1038/nrd4623
Farah C, Michel LYM, Balligand JL (2018) Nitric oxide signalling in cardiovascular health and disease. Nat Rev Cardiol 15(5):292–316. https://doi.org/10.1038/nrcardio.2017.224
Vong LB, Bui TQ, Tomita T, Sakamoto H, Hiramatsu Y, Nagasaki Y (2018) Novel angiogenesis therapeutics by redox injectable hydrogel - regulation of local nitric oxide generation for effective cardiovascular therapy. Biomaterials 167:143–152. https://doi.org/10.1016/j.biomaterials.2018.03.023
Qiu Y, Park K (2001) Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev 53(3):321–339
Shay JES, Simon MC (2012) Hypoxia-inducible factors: crosstalk between inflammation and metabolism. Semin Cell Dev Biol 23(4):389–394. https://doi.org/10.1016/j.semcdb.2012.04.004
Saito T, Tabata Y (2014) Hypoxia-induced angiogenesis is increased by the controlled release of deferoxiamine from gelatin hydrogels. Acta Biomater 10(8):3641–3649. https://doi.org/10.1016/j.actbio.2014.04.021
Hong Y, Zhou FF, Hua YJ, Zhang XZ, Ni CY, Pan DH, Zhang YQ, Jiang DM, Yang L, Lin QN, Zou YW, Yu DS, Arnot DE, Zou XH, Zhu LY, Zhang SF, Ouyang HW (2019) A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds. Nat Commun 10:ARTN 2060. https://doi.org/10.1038/s41467-019-10004-7
Leong DP, Joseph PG, McKee M, Anand SS, Teo KK, Schwalm JD, Yusuf S (2017) Reducing the global burden of cardiovascular disease, part 2 prevention and treatment of cardiovascular disease. Circ Res 121(6):695–710. https://doi.org/10.1161/Circresaha.117.311849
Funding
This study was funded by the Fostering Talents of National Natural Science Foundation of China and Henan Province (No. U1504310), Key Scientific Research Projects of Higher Education Institutions in Henan Province (No. 16A430030), Key Project and Special Foundation of Research, Development and Promotion in Henan Province (No. 182102310076), Top Doctor Program of Zhengzhou University (No. 32210475), and the Joint Fund for Fostering Talents of NCIR-MMT & HNKL-MMT (No. MMT2017-01).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guan, S., Li, J., Zhang, K. et al. Environmentally responsive hydrogels for repair of cardiovascular tissue. Heart Fail Rev 26, 1273–1285 (2021). https://doi.org/10.1007/s10741-020-09934-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-020-09934-y