Abstract
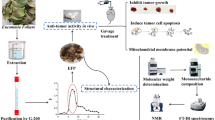
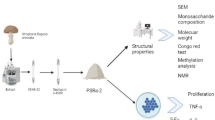
A soluble homogeneous β-glucan, GFPBW1, with a molecular mass of 300 kDa was purified from the fraction of the fruit bodies of Grifola frondosa extracted with 5 % NaOH. Using various methods, such as infrared spectroscopy, NMR, methylation and monosaccharide composition analysis, its structure was determined to be a β-D-(1-3)-linked glucan backbone with a single β-D-(1-6)-linked glucopyranosyl residue branched at C-6 on every third residue. It induced TNF-α and IL-6 production and the activation of Syk and NF-κB signaling in resident peritoneal macrophages from ICR mice, which could be significantly inhibited by the blocking reagent laminarin. A competitive phagocytosis assay with FITC-zymosan indicated that GFPBW1 could bind to DC-associated C-type lectin 1 (Dectin-1). The TNF-α secretion and activation of Syk/NF-κB signaling triggered by GFPBW1 were enhanced in RAW264.7 cells overexpressing wild but not mutant (Δ38 and Y15S) Dectin-1. Furthermore, GFPBW1 potentiated the Concanavalin A-induced proliferative response of splenocytes and inhibited Sarcoma-180 growth allografted in ICR mice but not in immunodeficient BALB/c nu/nu mice. These results suggested that the antitumor activity of GFPBW1 was partially associated with the activation of macrophages via the Dectin-1/Syk/NF-κB signaling pathway. This molecule could be a promising biological response modifier with clear application for antitumor therapies.






Similar content being viewed by others
Abbreviations
- BRMs:
-
Biological response modifiers
- CARD9:
-
Caspase recruitment domain 9
- ConA:
-
Concanavalin A
- CR3:
-
Complement receptor 3
- CTX:
-
Cyclophosphamide
- DCs:
-
Dendritic cells
- Dectin-1:
-
DC-associated C-type lectin 1
- DMSO:
-
Dimethyl sulfoxide
- FACS:
-
Fluorescence-activated cell sorting
- GC:
-
Gas chromatography
- G. frondosa :
-
Grifola frondosa (Fr.) S. F. Gray
- GRN:
-
Grifolan
- HPGPC:
-
High performance gel permeation chromatography
- HRP:
-
Horseradish peroxidase
- HSQC:
-
Heteronuclear single quantum coherence
- IR:
-
Infrared
- ITAM:
-
Immunoreceptor tyrosine-based activation motif
- LPS:
-
Lipopolysaccharide
- MS:
-
Mass spectrometry
- NF-κB:
-
Nuclear factor κB
- NMR:
-
Nuclear magnetic resonance
- PCR:
-
Polymerase chain reaction
- PMB:
-
Polymyxin B
- POD:
-
Peroxidase
- PRRs:
-
Pattern recognition receptors
- Syk:
-
Spleen tyrosine kinase
- TFA:
-
Trifluoroacetic acid
- TLR2:
-
Toll like receptor 2
- SDS:
-
Sodium dodecyl sulfate
References
Novak, M., Vetvicka, V.: Glucans as biological response modifiers. Endocr. Metab. Immune Disord. Drug Targets 9, 67–75 (2009)
Borchers, A.T., Keen, C.L., Gershwin, M.E.: Mushrooms, tumors, and immunity: an update. Exp. Biol. Med. (Maywood) 229, 393–406 (2004)
Mayell, M.: Maitake extracts and their therapeutic potential. Altern. Med. Rev. 6, 48–60 (2001)
Wasser, S.P.: Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 60, 258–274 (2002)
Ohno, N., Suzuki, I., Oikawa, S., Sato, K., Miyazaki, T., Yadomae, T.: Antitumor activity and structural characterization of glucans extracted from cultured fruit bodies of Grifola frondosa. Chem Pharm Bull (Tokyo) 32, 1142–1151 (1984)
Ohno, N., Iino, K., Takeyama, T., Suzuki, I., Sato, K., Oikawa, S., Miyazaki, T., Yadomae, T.: Structural characterization and antitumor activity of the extracts from matted mycelium of cultured Grifola frondosa. Chem. Pharm. Bull. (Tokyo) 33, 3395–3401 (1985)
Ohno, N., Adachi, Y., Suzuki, I., Sato, K., Oikawa, S., Yadomae, T.: Characterization of the antitumor glucan obtained from liquid-cultured Grifola frondosa. Chem. Pharm. Bull. (Tokyo) 34, 1709–1715 (1986)
Tada, R., Adachi, Y., Ishibashi, K., Ohno, N.: An unambiguous structural elucidation of a 1,3-beta-D-glucan obtained from liquid-cultured Grifola frondosa by solution NMR experiments. Carbohydr. Res. 344, 400–404 (2009)
Hashimoto, T., Ohno, N., Adachi, Y., Yadomae, T.: Nitric oxide synthesis in murine peritoneal macrophages by fungal beta-glucans. Biol. Pharm. Bull. 20, 1006–1009 (1997)
Masuda, Y., Togo, T., Mizuno, S., Konishi, M., Nanba, H.: Soluble beta-glucan from Grifola frondosa induces proliferation and Dectin-1/Syk signaling in resident macrophages via the GM-CSF autocrine pathway. J. Leukoc. Biol. (2011). doi:10.1189/jlb.0711386
Nanba, H., Hamaguchi, A., Kuroda, H.: The chemical structure of an antitumor polysaccharide in fruit bodies of Grifola frondosa (maitake). Chem. Pharm. Bull. (Tokyo) 35, 1162–1168 (1987)
Kodama, N., Asakawa, A., Inui, A., Masuda, Y., Nanba, H.: Enhancement of cytotoxicity of NK cells by D-Fraction, a polysaccharide from Grifola frondosa. Oncol. Rep. 13, 497–502 (2005)
Deng, G., Lin, H., Seidman, A., Fornier, M., D’Andrea, G., Wesa, K., Yeung, S., Cunningham-Rundles, S., Vickers, A.J., Cassileth, B.: A phase I/II trial of a polysaccharide extract from Grifola frondosa (Maitake mushroom) in breast cancer patients: immunological effects. J. Cancer Res. Clin. Oncol. 135, 1215–1221 (2009)
Brown, G.D., Gordon, S.: Fungal beta-glucans and mammalian immunity. Immunity 19, 311–315 (2003)
Goodridge, H.S., Wolf, A.J., Underhill, D.M.: Beta-glucan recognition by the innate immune system. Immunol. Rev. 230, 38–50 (2009)
Chen, J., Seviour, R.: Medicinal importance of fungal beta-(1– > 3), (1– > 6)-glucans. Mycol. Res. 111, 635–652 (2007)
Taylor, P.R., Tsoni, S.V., Willment, J.A., Dennehy, K.M., Rosas, M., Findon, H., Haynes, K., Steele, C., Botto, M., Gordon, S., Brown, G.D.: Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat. Immunol. 8, 31–38 (2007)
Rogers, N.C., Slack, E.C., Edwards, A.D., Nolte, M.A., Schulz, O., Schweighoffer, E., Williams, D.L., Gordon, S., Tybulewicz, V.L., Brown, G.D., Reis e Sousa, C.: Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22, 507–517 (2005)
Gantner, B.N., Simmons, R.M., Canavera, S.J., Akira, S., Underhill, D.M.: Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197, 1107–1117 (2003)
Drummond, R.A., Brown, G.D.: The role of Dectin-1 in the host defence against fungal infections. Curr. Opin. Microbiol. 14, 392–399 (2011)
Gross, O., Gewies, A., Finger, K., Schafer, M., Sparwasser, T., Peschel, C., Forster, I., Ruland, J.: Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 442, 651–656 (2006)
Masuda, Y., Ito, K., Konishi, M., Nanba, H.: A polysaccharide extracted from Grifola frondosa enhances the anti-tumor activity of bone marrow-derived dendritic cell-based immunotherapy against murine colon cancer. Cancer Immunol. Immunother. 59, 1531–1541 (2010)
Dong, Q., Jia, L.M., Fang, J.N.: A beta-D-glucan isolated from the fruiting bodies of Hericium erinaceus and its aqueous conformation. Carbohydr. Res. 341, 791–795 (2006)
Needs, P.W., Selvendran, R.R.: Avoiding Oxidative-Degradation during Sodium-Hydroxide Methyl Iodide-Mediated Carbohydrate Methylation in Dimethyl-Sulfoxide. Carbohyd. Res. 245, 1–10 (1993)
Kutner, R.H., Zhang, X.Y., Reiser, J.: Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat. Protoc. 4, 495–505 (2009)
Goodridge, H.S., Shimada, T., Wolf, A.J., Hsu, Y.M., Becker, C.A., Lin, X., Underhill, D.M.: Differential use of CARD9 by dectin-1 in macrophages and dendritic cells. J. Immunol. 182, 1146–1154 (2009)
Wu, X.M., Tu, P.F.: Isolation and characterization of alpha-(1– > 6)-glucans from Cistanche deserticola. J. Asian Nat. Prod. Res. 7, 823–828 (2005)
Yashoda, H.M., Prabha, T.N., Tharanathan, R.N.: Mango ripening–chemical and structural characterization of pectic and hemicellulosic polysaccharides. Carbohydr. Res. 340, 1335–1342 (2005)
Bao, X.F., Fang, J.N.: Structural characterization and chemical modification of a glucan from spores of Ganoderma lucidum. Chinese Chem. Lett. 12, 967–970 (2001)
Tada, R., Harada, T., Nagi-Miura, N., Adachi, Y., Nakajima, M., Yadomae, T., Ohno, N.: NMR characterization of the structure of a beta-(1– > 3)-D-glucan isolate from cultured fruit bodies of Sparassis crispa. Carbohydr. Res. 342, 2611–2618 (2007)
Mosser, D.M., Edwards, J.P.: Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008)
Adams, E.L., Rice, P.J., Graves, B., Ensley, H.E., Yu, H., Brown, G.D., Gordon, S., Monteiro, M.A., Papp-Szabo, E., Lowman, D.W., Power, T.D., Wempe, M.F., Williams, D.L.: Differential high-affinity interaction of dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side-chain branching. J. Pharmacol. Exp. Ther. 325, 115–123 (2008)
Brown, G.D., Taylor, P.R., Reid, D.M., Willment, J.A., Williams, D.L., Martinez-Pomares, L., Wong, S.Y., Gordon, S.: Dectin-1 is a major beta-glucan receptor on macrophages. J. Exp. Med. 196, 407–412 (2002)
Vallabhapurapu, S., Karin, M.: Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 27, 693–733 (2009)
Adachi, Y., Ishii, T., Ikeda, Y., Hoshino, A., Tamura, H., Aketagawa, J., Tanaka, S., Ohno, N.: Characterization of beta-glucan recognition site on C-type lectin, dectin 1. Infect. Immun. 72, 4159–4171 (2004)
Herre, J., Marshall, A.S., Caron, E., Edwards, A.D., Williams, D.L., Schweighoffer, E., Tybulewicz, V., Reis e Sousa, C., Gordon, S., Brown, G.D.: Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood 104, 4038–4045 (2004)
Kidd, P.M.: The use of mushroom glucans and proteoglycans in cancer treatment. Altern. Med. Rev. 5, 4–27 (2000)
Volman, J.J., Ramakers, J.D., Plat, J.: Dietary modulation of immune function by beta-glucans. Physiol. Behav. 94, 276–284 (2008)
Saijo, S., Fujikado, N., Furuta, T., Chung, S.H., Kotaki, H., Seki, K., Sudo, K., Akira, S., Adachi, Y., Ohno, N., Kinjo, T., Nakamura, K., Kawakami, K., Iwakura, Y.: Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat. Immunol. 8, 39–46 (2007)
Guo, L., Xie, J., Ruan, Y., Zhou, L., Zhu, H., Yun, X., Jiang, Y., Lu, L., Chen, K., Min, Z., Wen, Y., Gu, J.: Characterization and immunostimulatory activity of a polysaccharide from the spores of Ganoderma lucidum. Int. Immunopharmacol. 9, 1175–1182 (2009)
Okazaki, M., Adachi, Y., Ohno, N., Yadomae, T.: Structure-activity relationship of (1– > 3)-beta-D-glucans in the induction of cytokine production from macrophages, in vitro. Biol. Pharm. Bull. 18, 1320–1327 (1995)
Takeyama, T., Suzuki, I., Ohno, N., Oikawa, S., Sato, K., Ohsawa, M., Yadomae, T.: Host-mediated antitumor effect of grifolan NMF-5 N, a polysaccharide obtained from Grifola frondosa. J. Pharmacobiodyn. 10, 644–651 (1987)
Ikeda, Y., Adachi, Y., Ishii, T., Tamura, H., Aketagawa, J., Tanaka, S., Ohno, N.: Blocking effect of anti-Dectin-1 antibodies on the anti-tumor activity of 1,3-beta-glucan and the binding of Dectin-1 to 1,3-beta-glucan. Biol. Pharm. Bull. 30, 1384–1389 (2007)
Chan, G.C., Chan, W.K., Sze, D.M.: The effects of beta-glucan on human immune and cancer cells. J. Hematol. Oncol. 2, 25 (2009)
Liu, J., Gunn, L., Hansen, R., Yan, J.: Combined yeast-derived beta-glucan with anti-tumor monoclonal antibody for cancer immunotherapy. Exp. Mol. Pathol. 86, 208–214 (2009)
Acknowledgements
This work was supported by New Drug Creation and Manufacturing Program (2012ZX09301001-003), National Science Fund for Distinguished Young Scholars (81125025) and the funds for Industry-University-Research Institution Alliance in Guangdong Province, China (2010A090200041).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jianping Fang and Ying Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Fang, J., Wang, Y., Lv, X. et al. Structure of a β-glucan from Grifola frondosa and its antitumor effect by activating Dectin-1/Syk/NF-κB signaling. Glycoconj J 29, 365–377 (2012). https://doi.org/10.1007/s10719-012-9416-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-012-9416-z