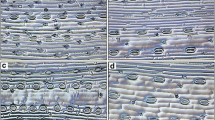
Abstract
Genotypic variation in stomatal density and size has been reported but little is known of the genetic mechanisms behind these leaf traits. Using 101 recombinant inbred lines derived from a cross between a tropical japonica, IR69093-41-3-2-2 and an indica variety, IR72, we conducted a field study to determine stomatal density and size and identify quantitative trait loci (QTL) controlling these traits under lowland conditions. Ten QTLs for stomatal density and four QTLs for size were identified across growth stages and leaf surfaces (adaxial and abaxial). The contribution of each QTL to total phenotypic variation ranged from 9.3 to 15.2% for stomatal density and 9.7 to 14.3% for size. The allele from IR72 increased stomatal density and that from IR69093-41-3-2-2 increased size. The expression of the QTLs for stomatal density and size differed by growth stage indicating that these traits might be genetically controlled depending on growth stage or that each QTL had a different function by growth stage. Significant negative genetic correlations between stomatal density and size at both vegetative (r = −0.308**) and heading (r = −0.484**) stages were observed but no common QTL for these traits was detected across growth stages and leaf surfaces. These results indicate that the QTLs for density and size may neither be genetically linked nor pleiotropically controlled and findings can be used as basis for selection at the leaf level on the balance of carbon and water uptake. Further study is needed to fully understand the mechanism underlying the observed genetic association and to elucidate the function of the QTLs involved.


Similar content being viewed by others
Abbreviations
- LOD:
-
Logarithm of the odds
- NPT:
-
New plant type
- QTL:
-
Quantitative trait loci
- RIL:
-
Recombinant inbred line
References
Aasamaa K, Sober A, Rahi M (2001) Leaf anatomical characteristics associated with shoot hydraulic conductance, stomatal conductance, and stomatal sensitivity to changes of leaf water status in temperate deciduous trees. Aust J Plant Physiol 28:765–774
Chen WF, Xu ZJ, Zhang LB, Yang SR (1990) Comparative studies on stomatal density and its relations to gas diffusion resistance and net photosynthetic rate in rice leaf. Chin J Rice Sci 4:63–168
Chen W, Xu ZJ, Qian TY, Zhang LB, Lee JY (1995) Comparative study of stomatal density and gas diffusion resistance in leaves of various types of rice. Korean J Crop Sci 40:125–132
Churchill GA, Doerge RW (1994) Empirical threshold values for quantitative trait mapping. Genetics 138:963–971
Ciha AJ, Brun WA (1974) Stomatal size and frequency in soybeans. Crop Sci 15:309–313
Dai Q, Peng S, Chavez AQ, Vergara BS (1995) Effects of UV-B radiation on stomatal density and opening in rice (Oryza sativa L.). Ann Bot 76:65–70
Fischer RA, Rees D, Sayre KD, Lu ZM, Condon AG, Saavedra AL (1998) Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci 38:1467–1475
Heichel GH (1970) Stomatal movements, frequencies, and resistances in two maize varieties differing in photosynthetic capacity. J Exp Bot 22:644–649
Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424:901–908
Ishihara K, Iida O, Hirasawa T (1979) Relationship between nitrogen content in leaf blades and photosynthetic rate of rice plants with reference to stomatal aperture and conductance. Jpn J Crop Sci 48:551–556
Ishimaru K, Shirota K, Higa M, Kawamitsu Y (2001) Identification of quantitative trait loci for adaxial and abaxial stomatal frequencies in Oryza sativa. Plant Physiol Biochem 39:173–177
Jones HG (1977) Transpiration in barley lines with differing stomatal frequencies. J Exp Bot 28:162–168
Jones HG (1998) Stomatal control of photosynthesis and transpiration. J Exp Bot 49:387–398
Kawamitsu Y, Agata W, Hiyane S, Murayama S, Nose A, Shinjyo C (1996) Relation between leaf gas exchange rate and stomata. Jpn J Crop Sci 65:626–633
Lander ES, Green P, Abrahamson J, Barlow A, Daley MJ (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage map of experimental and natural populations. Genomics 1:174–181
Laza MR, Kondo M, Ideta O, Barlaan E, Imbe T (2006) Identification of quantitative trait loci for δ13C and productivity in irrigated lowland rice. Crop Sci 46:763–773
Lu Z, Radin JW, Turcotte EL, Percy R, Zeiger E (1994) High yields in advanced lines of pima cotton are associated with stomatal conductance, reduced leaf area and lower leaf temperature. Physiol Plant 92:266–272
Maruyama S, Tajima K (1990) Leaf conductance in japonica and indica rice varieties. I. Size, frequency, and aperture of stomata. Jpn J Crop Sci 59:801–808
Miskin KE, Rasmusson DC (1970) Frequency and distribution of stomata in barley. Crop Sci 10:575–578
Miskin KE, Rasmusson DC, Moss DN (1972) Inheritance and physiological effects of stomatal frequency in barley. Crop Sci 12:781–783
Muchow RC, Sinclair TR (1989) Epidermal conductance, stomatal density and stomatal size among genotypes of Sorghum bicolor (L.) Moench. Plant Cell Environ 12:425–431
Oh YB, Lee DJ, Han HS, Park SH, Park RK (1988) Varietal differences in the frequency, size of stomata, and influence of nitrogen and low temperature on stomatal movement of rice seedlings. Korean Res Rep RDA 30:39–45
Ohsumi A, Kanemura T, Homma K, Horie T, Shiraiwa T (2007) Genotypic variation of stomatal conductance in relation to stomatal density and length in rice (Oryza sativa L.). Plant Prod Sci 10:322–328
Pearson M, Davies WJ, Mansfield TA (1995) Asymmetric responses of adaxial and abaxial stomata to elevated CO2: impacts on control of gas exchange by leaves. Plant Cell Environ 18:837–843
Peng S, Laza RC, Khush GS, Sanico AL, Visperas RM, Garcia FV (1998) Transpiration efficiencies of indica and improved tropical japonica rice grown under irrigated conditions. Euphytica 103:103–108
Pospisilova J, Solarova J (1980) Environmental and biological control of diffusive conductances of adaxial and abaxial leaf epidermis. Photosynthetica 14:90–127
Price AH, Cairns JE, Horton P, Jones HG, Griffiths G (2002) Linking drought-resistance mechanisms to drought avoidance in upland rice using a QTL approach: progress and new opportunities to integrate stomatal and mesophyll responses. J Exp Bot 53:989–1004
Reich PB (1984) Leaf stomatal density and diffusive conductance in three amphistomatous hybrid poplar cultivars. New Phytol 98:231–239
Romero-Aranda R, Canto-Garay R, Fernandez PF (1994) Distribution and density of stomata in two cultivars of Gerbera jamesonii and its relation to leaf conductance. Sci Hortic 58:167–173
Rowland-Bamford AJ, Nordenbrock C, Baker JT, Bowes G, Allen LH (1990) Changes in stomatal density in rice grown under various CO2 regimes with natural solar irradiance. Environ Exp Bot 30:175–180
SAS Institute Inc (2000–2004) SAS 9.1.3: help and documentation. SAS Institute Inc., Cary, NC
Schluter U, Muschak M, Berger D, Altmann T (2003) Photosynthetic performance of an Arabidopsis mutant with elevated stomatal density (sdd1–1) under different light regimes. J Exp Bot 54:867–874
Teare ID, Peterson CJ, Law AG (1971) Size and frequency of leaf stomata in cultivars of Triticum aestivum and other Triticum species. Crop Sci 11:496–498
Teng S, Qian Q, Zeng D, Kunihiro Y, Fujimoto K, Huang D, Zhu L (2004) QTL analysis of leaf photosynthetic rate and related physiological traits in rice (Oryza sativa L.). Euphytica 135:1–7
Tsunoda S, Fukoshima MT (1986) Leaf properties related to photosynthetic response to drought in upland and lowland varieties. Ann Bot 58:531–539
Walton PD (1974) The genetics of stomatal length and frequency in clones of Bromus inermis and the relationships between these traits and yield. Can J Plant Sci 54:749–754
Weng JH, Chen CY (1987) Stomatal frequency associated with an esterase band in rice genotypes. Rice Genet Newsl 5:93–95
Weyers JDB, Lawson TA (1997) Heterogeneity in stomatal characteristics. Adv Bot Res 26:317–352
Yang JC, Zhu QS, Wang ZQ, Lang YZ (1997) Photosynthetic characteristics, dry matter accumulation and its translocation in intersubspecific hybrid rice. Acta Agron Sin 23:82–88
Yoshida T (1979) Relationship between stomatal frequency and photosynthesis in barley. Jpn Agric Res Q 13:101–105
Yoshida T, Ono T (1978) Environmental differences in leaf stomatal frequency of rice. Jpn J Crop Sci 47:515–528
Zhang DP (1989) Studies of stomata on the rice plant leaf blade. II. Dynamic morphogenesis of stomata under varied ecological conditions. J Fujian Agric Coll 18:302–307
Acknowledgments
This work was partly supported by the Japan Society for Promotion of Science. We thank Dr. S. Koizumi (National Institute of Crop Science) for allowing the use of their microscope and Ms. Violeta I. Bartolome (Crop Research and Informatics Laboratory, IRRI) for her valuable assistance in statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laza, M.R.C., Kondo, M., Ideta, O. et al. Quantitative trait loci for stomatal density and size in lowland rice. Euphytica 172, 149–158 (2010). https://doi.org/10.1007/s10681-009-0011-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-009-0011-8