Abstract
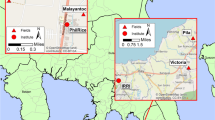
Ascochyta blight epidemics have been observed in many countries since the early 1900s but studies on an interaction between the amount of inoculum, environmental factors and the spatiotemporal development of Ascochyta blight are rare due to the historic emphasis on developing resistant cultivars and chemical control of the disease. I used generalised linear mixed models to investigate key environmental factors affecting the spatiotemporal development of Ascochyta blight from primary infection foci. Briefly, four replicate plots (20 m × 20 m) of a susceptible chickpea cultivar were planted at two different locations (Billa Billa and Tosari) in Queensland, Australia. Four naturally infested stubble pieces were placed at the centre of each newly emerged chickpea plot 14 days after sowing. The number of infected plants was counted in 1 m2 observation quadrats at the distances of 3, 6 and 9 m in a concentric arrangement. The number of infected plants increased with each assessment date, approaching 100% plant infections at the time of final assessment. The rate of disease progress was significantly faster at Tosari. The rate of disease progress significantly decreased as the distance from the primary infection foci increased. There was a significant positive effect of an optimum temperature, increasing rainfall and omni-directional wind. The influence of wind speed was not significant. The finding that single infection foci were enough to spread disease across whole plots indicate that limited inoculum is not a barrier in the development of an epiphytotic under conducive conditions.




Similar content being viewed by others
References
Akaike, H. (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19(6), 716–723.
Bar, I., Sambasivam, P. T., Davidson, J., Caceres, L. M. F., Lee, R. C., Hobson, K., et al. (2020). Current population structure and pathogenicity patterns of Ascochyta rabiei in Australia. bioRxiv. https://doi.org/10.1101/2020.12.21.423875.
Brooks, M. E., Kristensen, K., Van Benthem, K. J., Magnusson, A., Berg, C. W., Nielsen, A., et al. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal, 9(2), 378–400.
Chongo, G., Buchwaldt, L., Gossen, B., Lafond, G., May, W., Johnson, E., et al. (2003). Foliar fungicides to manage Ascochyta blight [Ascochyta rabiei] of chickpea in Canada. Canadian Journal of Plant Pathology, 25(2), 135–142.
Coventry, S. A. (2012). Factors affecting short and long distance dispersal of fungal pathogens: Chickpea Ascochyta blight as a model. PhD dissertation. The University of Adelaide, Australia.
Cubero, J. (1984). Ascochyta blight of chickpeas in Spain. In M. C. Saxena & K. B. Singh (Eds.), Ascochyta blight and winter sowing of chickpeas (pp. 273–281). Martinus Nijhoff/Dr. W. Junk Publishers.
Dey, S., & Singh, G. (1994). Seedborne infection of Ascochyta rabiei in chickpea and its transmission to aerial plant parts. Phytoparasitica, 22(1), 31–37.
FAOSTAT (2018). FAOSTAT database. Available at http://www.fao.org/faostat/en/-data/QC.
Gideon, S. (1978). Estimating the dimension of a model. The Annals of Statistics, 6(2), 461–464.
Griffiths, E., & Ao, H. C. (1976). Dispersal of Septoria nodorum spores and spread of glume blotch of wheat in the field. Transactions of the British Mycological Society, 67(3), 413–418.
Grolemund, G., & Wickham, H. (2011). Dates and times made easy with lubridate. Journal of Statistical Software, 40(3), 1–25.
Kaiser, W. (1973). Factors affecting growth, sporulation, pathogenicity, and survival of Ascochyta rabiei. Mycologia, 65(2), 444–457.
Kaiser, W. (1992). Epidemiology of Ascochyta rabiei. In K. Singh & M. Saxena (Eds.), Disease resistance breeding in chickpea (pp. 117–134). ICARDA.
Kaiser, W., & Küsmenoglu, I. (1997). Distribution of mating types and the teleomorph of Ascochyta rabiei on chickpea in Turkey. Plant Disease, 81(11), 1284–1287.
Ketelaer, E., Diekmann, M., & Weltzien, H. (1988). International spread of Ascochyta rabiei in chickpea seeds-an attempt at prognosis. International Chickpea Newsletter(18), 21-23.
Khaliq, I., Fanning, J., Melloy, P., Galloway, J., Moore, K., Burrell, D., & Sparks, A. H. (2020a). The role of conidia in the dispersal of Ascochyta rabiei. European Journal of Plant Pathology, 158, 911–924.
Khaliq, I., Fanning, J., Melloy, P., Galloway, J., Moore, K., Burrell, D., & Sparks, A. H. (2020b). The role of conidia in the dispersal of Ascochyta rabiei. European Journal of Plant Pathology, 158(4), 911–924.
Khan, M. S. A. (1999). Epidemiology of Ascochyta blight of chickpea in Australia. PhD dissertation. The University of Adelaide, Australia.
Kimber, R. (2002). Survival and transmission of Ascochyta rabiei (Ascochyta blight) of chickpea. Honours thesis, University of Adelaide, Australia.,
Kimber, R., Shtienberg, D., Ramsey, M., & Scott, E. (2007). The role of seedling infection in epiphytotics of Ascochyta blight on chickpea. European Journal of Plant Pathology, 117(2), 141–152.
Leo, A. E., Linde, C. C., & Ford, R. (2016). Defence gene expression profiling to Ascochyta rabiei aggressiveness in chickpea. Theoretical and Applied Genetics, 129(7), 1333–1345.
Madden, L., & Hughes, G. (1995). Plant disease incidence: Distributions, heterogeneity, and temporal analysis. Annual Review of Phytopathology, 33(1), 529–564.
Madden, L. V., Hughes, G., & Van Den Bosch, F. (2007). The study of plant disease epidemics. St. American Phytopathological Society.
Moore, K., Hobson, K., Sambasivam, P., Ford, R., Harden, S., Nash, P., et al. (2016). Chickpea Ascochyta - is the pathogen changing and what are the implications for management. Available at https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2015/02/chickpea-ascochyta-is-the-pathogen-changing-and-what-are-the-implications-for-management.
Navas-Cortés, J., Trapero-Casas, A., & Jiménez-Díaz, R. (1998). Influence of relative humidity and temperature on development of Didymella rabiei on chickpea debris. Plant Pathology, 47(1), 57–66.
Nene, Y. (1982). A review of Ascochyta blight of chickpea. International Journal of Pest Management, 28(1), 61–70.
Nene, Y., & Reddy, M. (1987). Chickpea diseases and their control. In M. Saxena & R. Singh (Eds.), The chickpea (pp. 233–270). CAB International.
Pande, S., Siddique, K., Kishore, G., Bayaa, B., Gaur, P., Gowda, C., et al. (2005). Ascochyta blight of chickpea (Cicer arietinum L.): A review of biology, pathogenicity, and disease management. Australian Journal of Agricultural Research, 56(4), 317–332.
Pedersen, E., Morrall, R., McCartney, H., & Fitt, B. D. (1994). Dispersal of conidia of Ascochyta fabae f. sp. lentis from infected lentil plants by simulated wind and rain. Plant Pathology, 43(1), 50–55.
Core Team, R. (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing.
Seers, B. M., & Shears, N. T. (2015). New Zealand’s climate data in R—An introduction to clifro. The University of Auckland http://stattech.wordpress.fos.auckland.ac.nz/2015-02-new-zealands-climate-data-in-r-an-introduction-to-clifro/.
Trapero-Casas, A., & Kaiser, W. (1992). Development of Didymella rabiei, the teleomorph of Ascochyta rabiei, on chickpea straw. Phytopathology, 82(11), 1261–1266.
Van Maanen, A., & Xu, X.-M. (2003). Modelling plant disease epidemics. European Journal of Plant Pathology, 109(7), 669–682.
Wallace, T. C., Murray, R., & Zelman, K. M. (2016). The nutritional value and health benefits of chickpeas and hummus. Nutrients, 8(12), 766.
Wickham, H., Averick, M., Bryan, J., Chang, W., McGowan, L., François, R., Grolemund, G., Hayes, A., Henry, L., Hester, J., Kuhn, M., Pedersen, T., Miller, E., Bache, S., Müller, K., Ooms, J., Robinson, D., Seidel, D., Spinu, V., Takahashi, K., Vaughan, D., Wilke, C., Woo, K., & Yutani, H. (2019). Welcome to the Tidyverse. Journal of Open Source Software, 4(43), 1686.
Xu, X.-M., & Ridout, M. (1998). Effects of initial epidemic conditions, sporulation rate, and spore dispersal gradient on the spatio-temporal dynamics of plant disease epidemics. Phytopathology, 88(10), 1000–1012.
Yang, X., Madden, L., Wilson, L., & Ellis, M. (1990). Effects of surface topography and rain intensity on splash dispersal of Colletotrichum acutatum. Phytopathology, 80(10), 1115–1120.
Bolker B (2021) GLMM FAQ. Available at https://bbolker.github.io/mixedmodelsmisc/glmmFAQ.html.
Vanderwal J, Falconi L, Januchowski S, Shoo L, Storlie C (2014) Species Distribution Modelling Tools, Tools for processingdata associated with species distribution modelling exercises. CRAN. R-project. org/package= SDMTools
Acknowledgments
This research was supported by Grains Research and Development Corporation (GRDC) through the project USQ1903-003-RTX. The application for the Postdoctoral Fellowship was put together by Adam H. Sparks (Department of Primary Industries and Regional Development) and Ihsanul Khaliq, which was supported by the national modelling project (Disease epidemiology and management tools for Australian grain growers-DAW1810-007RTX) national leader Jean Galloway and the project DSS leader Art Diggle (Department of Primary Industries and Regional Development) along with a valuable feedback on the grant application. I wish to acknowledge Queensland Department of Agriculture and Fisheries (QDAF) and MCA Agronomy Pty Ltd. staff for the trials planting and maintenance. I wish to thank Adam H. Sparks, Jenny Davidson (South Australian Research and Development Institute), Kevin Moore (NSW Department of Primary Industries) and Joshua Fanning (Agriculture Victoria) for their technical input on the experimental design. I also thank Andriamahery Rasolofoharivelo (University of Southern Queensland) and Andrew Erbacher (Queensland Department of Agriculture and Fisheries) for technical support.
Data availability and code availability
All raw and generated data used in the statistical analyses and data visualisation have been made available as a part of a research compendium for reproducibility and FAIR data policy. Please see the fully reproducible and version-controlled code at https://github.com/IhsanKhaliq/spatiotemporaldynamics. The archived code and data are available at https://doi.org/10.5281/zenodo.4563709
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study did not involve working with animals or humans.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
None to disclose.
Supplementary Information
ESM 1
(DOCX 260 kb)
Rights and permissions
About this article
Cite this article
Khaliq, I. Spatiotemporal development of Ascochyta blight in chickpea from primary infection foci: insights from plant, pathogen and the environment interactions to inform an epidemic risk. Eur J Plant Pathol 161, 331–342 (2021). https://doi.org/10.1007/s10658-021-02324-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-021-02324-6