Abstract
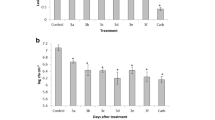
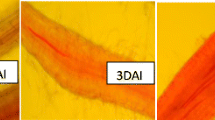
Three isomers of the ligand 2,5-bis(pyridinyl)-1,3,4-thiadiazole, with the N atom of pyridine group in position 2, 3 or 4, named respectively, L2, L3 and L4 were compared for their use as plant defense activators. They were examined for their ability to protect tomato plants from Verticillium dahliae and Agrobacterium tumefaciens in the greenhouse, to induce reactive oxygen species and to activate plant defenses, including antioxidant enzymes. The three ligand isomers exhibited in vitro only slight inhibition of radial growth of V. dahliae, while no significant inhibition was observed for phytopathogenic bacteria. In the greenhouse, the three ligand isomers statistically reduced the severity of Verticillium wilt and crown gall on tomato plants, and the isomers L3 and L4 were the most efficient to control Verticillium wilt. This superiority was reflected in their differential ability to activate H2O2 accumulation, antioxidant enzymes including catalase and ascorbate peroxidase and other defense-related enzymes such as guaiacol peroxidase and polyphenol oxidase. These results demonstrated that the presence of the N atom within the two pyridinyl groups in the position 3 or 4 highly enhanced the activity of plant defense and antioxidant responses as well as their ability to reduce the severity of symptoms caused by V. dahliae on tomato.









Similar content being viewed by others
References
Alexieva, V., Sergiev, I., Mapelli, S., & Karanov, E. (2001). The effect of drought and ultraviolet radiation growth and stress markers in pea and wheat. Plant Cell Environment, 24, 1337–1344.
AL-Quraan, N. A., Al-Smadi, M. L., & Swaleh, A. F. (2015). GABA metabolism and ROS induction in lentil (Lens culinaris Medik) plants by synthetic 1,2,3-thiadiazole compounds. Journal of Plant Interactions, 10, 185–194.
Becke, A. D. (1993). Density-functional thermochemistry. III. The role of exact exchange. The Journal of Chemical Physics, 98, 5648–5652.
Bektas, Y., & Eulgem, T. (2015). Synthetic plant defense elicitors. Frontiers in Plant Sciences, 5. https://doi.org/10.3389/fpls.2014.00804.
Chawla, A., Chawla, P., & Baghel, U. S. (2014). Synthesis and biological evaluation of thiazole-thiadiazole bridged compounds. Asian Journal of Biochemical and Pharmaceutical Research, 4, 269–275.
Chen, H. S., Li, Z. M., Han, Y. F., & Wang, Z. W. (1999). New fungicidally active pyrazolyl-substituted 1,3,4-thiadiazole compounds and their preparation. Chinese Chemistry Letters, 10, 365–366.
Chen, C. J., Song, B. A., Yang, S., Xu, G. F., Bhadury, P. S., Jin, L. H., Hu, D. Y., Li, Q. Z., Liu, F., Xue, W., Lu, P., & Chen, Z. (2007). Synthesis and antifungal activities of 5-(3,4,5- trimethoxyphenyl)-2-sulfonyl-1,3,4-thiadiazole and 5- (3,4,5-trimethoxy phenyl)-2-sulfonyl-1,3,4-oxadiazole derivatives. Bioorganic & Medicinal Chemistry, 15, 3981–3989.
Cherrab, M., Bennani, A., Charest, P. M., & Serrhini, M. N. (2002). Pathogenecity and vegetative compatibility of Verticillium dahlia Kleb. Isolates from olives in Morocco. Journal of Phytopathology, 150(11–12), 703–709.
Conrath, U., Beckers, G. J. M., Flors, V., García-Agustín, P., Jakab, G., Mauch, F., Newman, M. A., Pieterse, C. M. J., Poinssot, B., Pozo, M. J., Pugin, A., Schaffrath, U., Jurriaan, T., Wendehenne, D., Zimmerli, L., & Mauch-Mani, B. (2006). Priming: Getting ready for battle. Molecular Plant-Microbe Interactions, 19(10), 1062–1071.
Daayf, F., Nicole, M., & Geiger, J. P. (1995). Differentiation of Verticillium dahlia on the basis of vegetative compatibility and pathogenicity on cotton. European Journal of Plant Pathology, 101, 69–79.
Deepak, S. A., Ishii, H., & Park, P. (2006). Acibenzolar-S-methyl primes cell wall strengthening genes and reactive oxygen species forming/scavenging enzymes in cucumber after fungal pathogen attack. Physiological and Molecular Plant Pathology, 69, 52–59.
Elmeligie, S., Abdalla Khalil, N., Mohamed Ahmed, E., Emam, S. H., & Zaitone, S. A. (2016). Synthesis of new N1-substituted-5-aryl-3-(3,4,5-trimethoxyphenyl)-2-pyrazoline derivatives as antitumor agents targeting the colchicine site on tubulin. Biological and Pharmaceutical Bulletin, 39, 1611–1622.
Faize, M., Faize, L., Koike, N., Ishizaka, M., & Ishii, H. (2004). Acibenzolar-S-methyl-induced resistance to Japanese pear scab is associated with potentiation of multiple defense responses. Phytopathology, 94, 604–612.
Faize, M., Faize, L., & Ishii, H. (2009). Gene expression during acibenzolar-S-methyl-induced priming for potentiated responses to Venturia nashicola in Japanese pear. Journal of Phytopathology, 157, 137–144.
Faize, M., Burgos, L., Faize, L., Petri, C., Barba-Espin, G., Diaz-Vivancos, P., Clemente-Moreno, M. J., Alburquerque, N., & Hernandez, J. A. (2012). Modulation of tobacco bacterial disease resistance using cytosolic ascorbate peroxidase and cu/Zn superoxide dismutase. Plant Pathology, 61, 858–866.
Foyer, C. H., & Noctor, G. (2005). Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell, 17, 1866–1875.
Iwata, M. (2001). Probenazole-a plant defence activator. Pesticide Outlook, 12, 28–31.
Kadota, Y., Shirasu, K., & Zipfel, C. (2015). Regulation of the NADPH oxidase RBOHD during plant immunity. Plant Cell Physiology, 56, 1472–1480.
Kessmann, H., Staub, T., Hofmann, C., Maetzke, T., Herzog, J., Ward, E., Uknes, S., & Ryals, J. (1994). Induction of systemic acquired resistance in plants by chemicals. Annual Review of Phytopathology, 32, 439–459.
King, E. O., Ward, M. K., & Raney, D. E. (1954). Two simple media for the demonstration of pyocyanin and fluorescein. Journal of Laboratory and Clinical Medicine, 44, 301–307.
Klosterman, S. J., Subbarao, K. V., Kang, S., Veronese, P., Gold, S. E., et al. (2011). Comparative genomics yields insights into niche adaptation of plant vascular wilt pathogens. PLoS Pathogens, 7, e1002137.
Kubo, H., Sato, R., Hamura, I., & Ohi, T. (1970). Herbicidal activity of 1,3,4-thiadiazole derivatives. Journal of Agriculture and Food Chemistry, 18, 60–65.
Kumar, J. A., Sharma, S., Vaidya, A., Ravichandran, V., & Agrawal, R. K. (2013). 1,3,4-thiadiazole and its derivatives: A review on recent progress in biological activities. Chemical Biology Drug Design, 81, 557–576.
Kuzniak, E., & Urbanek, H. (2000). The involvement of hydrogen peroxide in plant responses to stresses. Acta Physiologica Plantarum, 22, 195–203.
Lebrini, M., Bentiss, F., & Legrenée, M. (2005). Rapid synthesis of 2,5-disubtituted 1,3,4-thiadiazoles under microwave irradiation. Journal of Heterocyclic Chemistry, 42, 991–994.
Lee, C., Yang, W., & Parr, R. G. (1988). Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Physical Review B, 37, 785–789.
Li, L., & Steffens, J. C. (2002). Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta, 215, 239–247.
Masia, A., Ventura, M., Gemma, H., & Sansavini, S. (1998). Effect of some plant growth regulator treatments on apple fruit ripening. Plant Growth Regulation, 25, 127–134.
Mayer, A. M. (2002). Polyphenol oxidases in plants and fungi: Going places? Phytochemistry, 67, 2318–2331.
Mishra, G., Singh, A. K., & Jyoti, K. (2011). Review article on 1,3,4-thiadiazole derivaties and it’s pharmacological activities. International Journal of ChemTech Research, 3, 1380–1393.
Moerschbacher, B., Heck, B., Kogel, K. H., Obst, O., & Reisener, H. G. (1986). An elicitor of the hypersensitive lignification response in wheat leaves isolated from the rust fungus Puccinia graminis f. sp. tritici. II. Induction of enzymes correlated with the biosynthesis of lignin. Zeitschrift fur Naturforschung 41c, 839-844.
Oostendorp, M., Kunz, W., Dietrich, B., & Staub, T. (2001). Induced disease resistance in plants by chemicals. European Journal of Plant Pathology, 107, 19–28.
Pitzschke, A. (2013). Agrobacterium infection and plant defense - transformation success hangs by a thread. Frontiers in Plant Science, 4, 519. https://doi.org/10.3389/fpls.2013.00519.
Quiroga, M., Guerrero, C., Botella, M. A., Barcelo, A., Amaya, I., Medina, M. I., Alonso, F. J., de Forchetti, S. M., Tigier, H., & Valpuesta, V. (2000). A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiology, 122, 1119–1127.
Smaili, A., Mazoir, N., Rifai, L. A., Koussa, T., Makroum, K., Benharref, A., Faize, L., Alburquerque, N., Burgos, L., Belfaiza, M., & Faize, M. (2017). Antimicrobial activity of two semisynthetic triterpene derivatives from Euphorbia officinarum latex against fungal and bacterial phyotopathogens. Natural Product Communications, 12, 331–336.
Swamy, S. N., Basappa, Priya, B. S., Prabhuswamy, B., Doreswamy, B. H., Prasad, J. S., & Rangappa, K. S. (2006). Synthesis of pharmaceutically important condensed heterocyclic 4,6-disubstituted-1,2,4-triazolo-1,3,4-thiadiazole derivatives as antimicrobials. European Journal of Medicinal Chemistry, 41, 531–538.
Tahghighi, A., & Babalouei, F. (2017). Thiadiazoles: The appropriate pharmacological scaffolds with leishmanicidal and antimalarial activities: A review. Iranian Journal of Basic Medical Sciences, 20, 613–622.
Yasuda, M., Nakashita, H., & Yoshida, S. (2004). Tiadinil, a novel class of activator of systemic acquired resistance, induces defense gene expression and disease resistance in tobacco. Journal of Pesticide Science, 29, 46–49.
Yasuda, M., Kusajima, M., Nakajima, M., Akutsu, K., Kudo, T., Yoshida, S., et al. (2006). Thiadiazole carboxylic acid moiety of tiadinil, sv-03, induces systemic acquired resistance in tobacco without salicylic acid accumulation. Journal of Pesticide Science, 31, 329–334.
Zine, H., Rifai, L. A., Faize, M., Smaili, A., Makroum, K., Belfaiza, M., Kabil, E. M., & Koussa, T. (2016). Duality of acibenzolar-S-methyl in the inhibition of pathogen growth and induction of resistance during the interaction tomato / Vertcillium dahliae. European Journal of Plant Pathology, 145, 61–69.
Zine, H., Rifai, L. A., Koussa, T., Bentiss, F., Guesmi, S., Laachir, A., Makroum, K., Belfaiza, M., & Faize, M. (2017). The mononuclear nickel (II) complex bis(azido-kN)bis[2,5-bis(pyridin-2-yl)-1,3,4-thiadiazole-κ(2) N(2) ,N(3) ]nickel(II) protects tomato from Verticillium dahliae by inhibiting the fungal growth and activating plant defenses. Pesticide Managment Science, 73, 188–197.
Zucconi, F., Pera, A., Forte, M., & De Bertoli, M. (1981). Evaluating toxicity in immature compost. Biocycle, 22, 54–57.
Acknowledgments
The authors gratefully acknowledge the CUR CA2D of Chouaib Doukkali University (El Jadida-Morocco) for its support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state they have no conflict of interest.
Ethical approval
This research does not contain any studies with human or animal subjects. The data presented in the article are original and have not been published in the public. All authors have written, read and consented to the manuscript.
Rights and permissions
About this article
Cite this article
Smaili, A., Laachir, A., Rifai, L.A. et al. Effect of altering the steric hindrance of the nitrogen atom in 2,5-bis(pyridin-n-yl)-1,3,4 thiadiazole isomers on their ability to elicit tomato defense responses against Verticillium wilt and crown gall diseases. Eur J Plant Pathol 151, 1035–1048 (2018). https://doi.org/10.1007/s10658-018-1441-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-018-1441-8