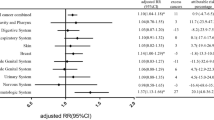
Abstract
Most of the pooled analyses and reviews reported an association between radiotherapy for childhood cancer and an increased thyroid cancer risk. Up to now this article presents the first systematic literature review on this association combined with a critical assessment of the methodological quality of the included articles. PubMed and Web of Science databases were searched for relevant articles until May 2016. We included peer-reviewed cohort and case–control studies that investigated an association between radiotherapy for childhood cancer and the occurrence of subsequent thyroid cancer. A systematic overview is presented for the included studies. We identified 17 retrospective cohort studies, and four nested case–control studies, representing 100,818 subjects. The age range at first cancer diagnosis was 0–25.2 years. Considerable variability was found regarding study sizes, study design, treatment strategies, dose information, and follow-up periods. 20 of the 21 identified studies showed increased thyroid cancer risks associated with childhood radiation exposure. The large majority showed an increased relative risk or odds ratio confirming the association between radiotherapy and thyroid cancer although the variation in results was large. Additionally to a pooled analysis that has been published recently, we systematically included 17 further studies, which allowed us to cover information from countries that were not covered by large-scale childhood cancer survivor studies. The methodological limitations of existing studies and inconsistencies in findings across studies yielded a large study heterogeneity, which made a detailed comparison of study results difficult. There is a need to strengthen standardisation for reporting.



Similar content being viewed by others
References
Bhatti P, Veiga LH, Ronckers CM, et al. Risk of second primary thyroid cancer after radiotherapy for a childhood cancer in a large cohort study: an update from the childhood cancer survivor study. Radiat Res. 2010;174(6):741–52. https://doi.org/10.1667/rr2240.1.
Haddy N, Andriamboavonjy T, Paoletti C, et al. Thyroid adenomas and carcinomas following radiotherapy for a hemangioma during infancy. Radiother Oncol. 2009;93(2):377–82. https://doi.org/10.1016/j.radonc.2009.05.011.
Sigurdson AJ, Ronckers CM, Mertens AC, et al. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): a nested case-control study. Lancet. 2005;365(9476):2014–23. https://doi.org/10.1016/s0140-6736(05)66695-0.
Ron E, Brenner A. Non-malignant thyroid diseases after a wide range of radiation exposures. Radiat Res. 2010;174(6):877–88. https://doi.org/10.1667/rr1953.1.
Veiga LH, Holmberg E, Anderson H, et al. Thyroid cancer after childhood exposure to external radiation: an updated pooled analysis of 12 studies. Radiat Res. 2016;185(5):473–84. https://doi.org/10.1667/rr14213.1.
La Vecchia C, Malvezzi M, Bosetti C, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer. 2015;136(9):2187–95. https://doi.org/10.1002/ijc.29251.
Mullenders L, Atkinson M, Paretzke H, Sabatier L, Bouffler S. Assessing cancer risks of low-dose radiation. Nat Rev Cancer. 2009;9(8):596–604.
Kumar S. Second malignant neoplasms following radiotherapy. Int J Env Res Public Health. 2012;9(12):4744–59. https://doi.org/10.3390/ijerph9124744.
Furukawa K, Preston D, Funamoto S, et al. Long-term trend of thyroid cancer risk among Japanese atomic-bomb survivors: 60 years after exposure. Int J Cancer. 2013;132(5):1222–6. https://doi.org/10.1002/ijc.27749.
Douple EB, Mabuchi K, Cullings HM, et al. Long-term radiation-related health effects in a unique human population: lessons learned from the atomic bomb survivors of Hiroshima and Nagasaki. Disaster Med Public Health Prep. 2011;5(1):S122–33. https://doi.org/10.1001/dmp.2011.21.
Sachs RK, Brenner DJ. Solid tumor risks after high doses of ionizing radiation. Proc Natl Acad Sci USA. 2005;102(37):13040–5. https://doi.org/10.1073/pnas.0506648102.
de Vathaire F, Hardiman C, Shamsaldin A, et al. Thyroid carcinomas after irradiation for a first cancer during childhood. Arch Intern Med. 1999;159(22):2713–9.
Prise KM, Schettino G, Folkard M, Held KD. New insights on cell death from radiation exposure. Lancet Oncol. 2005;6(7):520–8. https://doi.org/10.1016/s1470-2045(05)70246-1.
Armenian SH, Landier W, Hudson MM, Robison LL, Bhatia S. Children’s Oncology Group’s 2013 blueprint for research: survivorship and outcomes. Pediatr Blood Cancer. 2013;60(6):1063–8. https://doi.org/10.1002/pbc.24422.
Gatta G, Botta L, Rossi S, et al. Childhood cancer survival in Europe 1999-2007: results of EUROCARE-5—a population-based study. Lancet Oncol. 2014;15(1):35–47. https://doi.org/10.1016/s1470-2045(13)70548-5.
Berrington de Gonzalez A, Gilbert E, Curtis R, et al. Second solid cancers after radiation therapy: a systematic review of the epidemiologic studies of the radiation dose-response relationship. Int J Radiat Oncol Biol Phys. 2013;86(2):224–33. https://doi.org/10.1016/j.ijrobp.2012.09.001.
Scholz-Kreisel P, Spix C, Blettner M, et al. Prevalence of cardiovascular late sequelae in long-term survivors of childhood cancer: a systematic review and meta-analysis. Pediatr Blood Cancer. 2017. https://doi.org/10.1002/pbc.26428.
2013 UR. Scientific annex B: effects of radiation exposure of children. New York: United Nations; 2013.
Ron E, Lubin JH, Shore RE, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res. 1995;141(3):259–77.
Veiga LH, Lubin JH, Anderson H, et al. A pooled analysis of thyroid cancer incidence following radiotherapy for childhood cancer. Radiat Res. 2012;178(4):365–76.
Lundell M, Hakulinen T, Holm LE. Thyroid cancer after radiotherapy for skin hemangioma in infancy. Radiat Res. 1994;140(3):334–9.
Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–6. https://doi.org/10.7326/0003-4819-158-4-201302190-00009.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–9. https://doi.org/10.1016/j.ijsu.2014.07.013.
Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23(9):1351–75. https://doi.org/10.1002/sim.1761.
Sankey SS, Weissfeld LA, Fine MJ, Kapoor W. An assessment of the use of the continuity correction for sparse data in meta-analysis. Commun Stat Simul Comput. 1996;25(4):1031–56. https://doi.org/10.1080/03610919608813357.
Svahn-Tapper G, Garwicz S, Anderson H, et al. Radiation dose and relapse are predictors for development of second malignant solid tumors after cancer in childhood and adolescence: a population-based case-control study in the five Nordic countries. Acta Oncol. 2006;45(4):438–48. https://doi.org/10.1080/02841860600658633.
Tucker MA, Jones PHM, Boice JD, et al. Therapeutic radiation at a young age is linked to secondary thyroid cancer. Cancer Res. 1991;51(11):2885–8.
Finke I, Scholz-Kreisel P, Hennewig U, Blettner M, Spix C. Radiotherapy and subsequent thyroid cancer in German childhood cancer survivors: a nested case-control study. Radiat Oncol. 2015;10:219. https://doi.org/10.1186/s13014-015-0521-6.
Broniscer A, Ke W, Fuller CE, Wu J, Gajjar A, Kun LE. Second neoplasms in pediatric patients with primary central nervous system tumors: the St. Jude Children’s Research Hospital experience. Cancer. 2004;100(10):2246–52. https://doi.org/10.1002/cncr.20253.
Navid F, Billups C, Liu T, Krasin MJ, Rodriguez-Galindo C. Second cancers in patients with the Ewing sarcoma family of tumours. Eur J Cancer. 2008;44(7):983–91. https://doi.org/10.1016/j.ejca.2008.02.027.
Nygaard R, Garwicz S, Haldorsen T, et al. Second malignant neoplasms in patients treated for childhood leukemia: a population-based cohort study from the Nordic countries. Acta Paediatr. 1991;80(12):1220–8. https://doi.org/10.1111/j.1651-2227.1991.tb11812.x.
Bhatia S, Sather HN, Pabustan OB, Trigg ME, Gaynon PS, Robison LL. Low incidence of second neoplasms among children diagnosed with acute lymphoblastic leukemia after 1983. Blood. 2002;99(12):4257–64.
Bhatia S, Yasui Y, Robison LL, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin’s disease: report from the Late Effects Study Group. J Clin Oncol. 2003;21(23):4386–94. https://doi.org/10.1200/jco.2003.11.059.
Chow EJ, Friedman DL, Stovall M, et al. Risk of thyroid dysfunction and subsequent thyroid cancer among survivors of acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2009;53(3):432–7. https://doi.org/10.1002/pbc.22082.
Inskip PD, Curtis RE. New malignancies following childhood cancer in the United States, 1973–2002. Int J Cancer. 2007;121(10):2233–40. https://doi.org/10.1002/ijc.22827.
Lee JS, DuBois SG, Coccia PF, Bleyer A, Olin RL, Goldsby RE. Increased risk of second malignant neoplasms in adolescents and young adults with cancer. Cancer. 2016;122(1):116–23. https://doi.org/10.1002/cncr.29685.
Metayer C, Lynch CF, Clarke EA, et al. Second cancers among long-term survivors of Hodgkin’s disease diagnosed in childhood and adolescence. J Clin Oncol. 2000;18(12):2435–43. https://doi.org/10.1200/jco.2000.18.12.2435.
Rose J, Wertheim BC, Guerrero MA. Radiation treatment of patients with primary pediatric malignancies: risk of developing thyroid cancer as a secondary malignancy. Am J Surg. 2012;204(6):881–6. https://doi.org/10.1016/j.amjsurg.2012.07.030 (discussion 6–7).
Socie G, Curtis RE, Deeg HJ, et al. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia. J Clin Oncol. 2000;18(2):348–57. https://doi.org/10.1200/jco.2000.18.2.348.
Rubino C, Adjadj E, Guerin S, et al. Long-term risk of second malignant neoplasms after neuroblastoma in childhood: role of treatment. Int J Cancer. 2003;107(5):791–6. https://doi.org/10.1002/ijc.11455.
Dorffel W, Riepenhausenl M, Luders H, Bramswig J, Schellong G. Secondary malignancies following treatment for Hodgkin’s lymphoma in childhood and adolescence. Dtsch Arztebl Int. 2015;112(18):320–7. https://doi.org/10.3238/arztebl.2015.0320.
Cohen A, Rovelli A, van Lint MT, et al. Secondary thyroid carcinoma after allogeneic bone marrow transplantation during childhood. Bone Marrow Transplant. 2001;28(12):1125–8. https://doi.org/10.1038/sj.bmt.1703290.
de Vathaire F, Francois P, Schlumberger M, et al. Epidemiological evidence for a common mechanism for neuroblastoma and differentiated thyroid tumour. Br J Cancer. 1992;65(3):425–8.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. https://doi.org/10.1016/j.ijsu.2010.02.007.
Higgins J, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011.
Freycon C, Berger C, Casagranda L, et al. Effets tardifs de la radiothérapie pour un cancer dans l’enfance traité entre 1987 et 1992 en région Auvergne-Rhône-Alpes: résultats de l’étude SALTO. Rev Oncol Hématol Pédiatr. 2016;4(4):210–21. https://doi.org/10.1016/j.oncohp.2016.10.002.
Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9(3):193–9. https://doi.org/10.7150/ijms.3635.
Bhide SA, Nutting CM. Recent advances in radiotherapy. BMC Med. 2010;8(1):25. https://doi.org/10.1186/1741-7015-8-25.
Lubin JH, Adams MJ, Shore R, et al. Thyroid cancer following childhood low dose radiation exposure: a pooled analysis of nine cohorts. J Clin Endocrinol Metab. 2017. https://doi.org/10.1210/jc.2016-3529.
Acknowledgements
The authors would like to thank the Deutsche Krebshilfe (German Cancer Aid) for supporting this work (Award Number: 70111916). The content of this publication is solely the responsibility of the authors.
Author information
Authors and Affiliations
Contributions
MB, PSK and RP wrote the grant application and laid down the overall principles of the study, and advised on conceptual issues. EL compiled the search. EL, PSK, and DB screened titles, abstracts and full-text articles for inclusion, and completed the quality and risk of bias assessment. EL led the review process, the analysis and the writing. All authors read, edited and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix
PubMed and Web of Science search strategy conducted on May 20th, 2016
(Child OR Child* OR Pediatric OR Adolescent OR Infant) AND (cancer OR neoplasm OR tumor OR malignancy OR hemangioma) AND (radiation therapy OR irradiation OR radiation treatment OR radiotherapy) AND ((Second OR subsequent OR secondary OR second primary) AND (Thyroid Neoplasm OR Thyroid Cancer)).
Rights and permissions
About this article
Cite this article
Lorenz, E., Scholz-Kreisel, P., Baaken, D. et al. Radiotherapy for childhood cancer and subsequent thyroid cancer risk: a systematic review. Eur J Epidemiol 33, 1139–1162 (2018). https://doi.org/10.1007/s10654-018-0467-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-018-0467-8