Summary
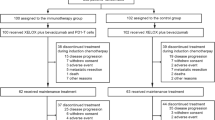
Background Purpose of this phase Ib trial was to establish the maximum tolerable dose (MTD) of capecitabine and to escalate the dosages of erlotinib and bevacizumab to determine the recommended phase II dose (RP2D) in patients with advanced/metastatic pancreatic adenocarcinoma not pretreated for metastatic disease. Methods Starting doses were capecitabine 500 mg/m2 bid orally continuously, erlotinib 100 mg orally daily, and bevacizumab 5 mg/kg intravenously q 2 weeks. Dose escalation was performed according to a 3 + 3 design for capecitabine until MTD, for erlotinib and bevacizumab until the maximum doses registered by applying a substance-related, toxicity-based scheme accompanied by pharmacokinetic analysis. Circulating tumor cells (CTCs) were determined pretherapeutically by immunohistochemical identification after enrichment with immunomagnetic separation. Results Thirty patients were evaluable at six dose levels. 900 mg/m2 bid were determined as MTD for capecitabine based on dose-limiting toxicities: cutaneous in two patients and vascular in another. The most severe (Grade (G)3/4) drug-related treatment-emergent adverse events (toxicities) belonged to the categories gastrointestinal, vascular, cutaneous, cardiovascular, metabolic/nutritional or hematological. G3 toxicities occurred in 14 (47%), G3 + G4 in a single (3%) patient. 2 out of 28 patients (7%) exerted partial response, 17 (61%) stable disease. Pharmacokinetic evaluation revealed lack of drug-drug interaction between capecitabine and erlotinib and their metabolites. Presence of CTCs was associated with shorter progression-free survival (p = 0.009). Conclusions The study met the primary objective. RP2D was capecitabine 800 mg/m2 bid continuously, erlotinib 150 mg daily, and bevacizumab 10 mg/kg q 2 weeks. The regimen could be applied safely, but demonstrated limited efficacy.


Similar content being viewed by others
References
Dreyer SB, Chang DK, Bailey P, Biankin AV (2017) Pancreatic cancer genomes: implications for clinical management and therapeutic development. Clin Cancer Res 23:1638–1646. https://doi.org/10.1158/1078-0432.CCR-16-2069
Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15:2403–2413
Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M, for the Groupe Tumeurs Digestives of Unicancer and PRODIGE Intergroup (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825. https://doi.org/10.1056/NEJMoa1011923
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369:1691–1703. https://doi.org/10.1056/NEJMoa1304369
Welch SA, Moore MJ (2007) Combination chemotherapy in advanced pancreatic cancer: time to raise the white flag? J Clin Oncol 25:2159–2161
Bates SE (2017) Pancreatic cancer: challenge and inspiration. Clin Cancer Res 23:1628. https://doi.org/10.1158/1078-0432.CCR-16-2069
Meta-analysis Group in Cancer, Piedbois P, Rougier P, Buyse M, Pignon J, Ryan L, Hansen R, Zee B, Weinerman B, Pater J, Leichman C, Macdonald J, Benedetti J, Lokich J, Fryer J, Brufman G, Isacson R, Laplanche A, Levy E (1998) Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol 16:301–308
Budman DR, Meropol NJ, Reigner B, Creaven PJ, Lichtman SM, Berghorn E, Behr J, Gordon RJ, Osterwalder B, Griffin T (1998) Preliminary studies of a novel oral fluoropyrimidine carbamate: capecitabine. J Clin Oncol 16:1795–1802
Van Cutsem E, Findlay M, Osterwalder B, Kocha W, Dalley D, Pazdur R, Cassidy J, Dirix L, Twelves C, Allman D, Seitz JF, Schölmerich J, Burger HU, Verweij J (2000) Capecitabine, an oral fluoropyrimidine carbamate with substantial activity in advanced colorectal cancer: results of a randomized phase II study. J Clin Oncol 18:1337–1345
Friess H, Wang L, Zhu Z, Gerber R, Schröder M, Fukuda A, Zimmermann A, Korc M, Büchler MW (1999) Growth factor receptors are differentially expressed in cancers of the papilla of vater and pancreas. Ann Surg 230:767–774
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W, National Cancer Institute of Canada Clinical Trials Group (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada clinical trials group. J Clin Oncol 25:1960–1966
Korc M (2003) Pathways for aberrant angiogenesis in pancreatic cancer. Mol Cancer 2:1–8
Kindler HL, Friberg G, Singh DA, Locker G, Nattam S, Kozloff M, Taber DA, Karrison T, Dachman A, Stadler WM, Vokes EE (2005) Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol 23:8033–8040
Tabernero J (2007) The role of VEGF and EGFR inhibition: implications for combining anti-VEGF and anti-EGFR agents. Mol Cancer Res 5:203–220
Ciardiello F, Bianco R, Damiano V, Fontanini G, Caputo R, Pomatico G, De Placido S, Bianco AR, Mendelsohn J, Tortora G (2000) Antiangiogenic and antitumor activity of anti-epidermal growth factor receptor C225 monoclonal antibody in combination with vascular endothelial growth factor antisense oligonucleotide in human GEO colon cancer cells. Clin Cancer Res 6:3739–3747
Jung YD, Mansfield PF, Akagi M, Takeda A, Liu W, Bucana CD, Hicklin DJ, Ellis LM (2002) Effects of combination anti-vascular endothelial growth factor receptor and anti-epidermal growth factor receptor therapies on the growth of gastric cancer in a nude mouse model. Eur J Cancer 38:1133–1140
Pantel K, Brakenhoff RH, Brandt B (2008) Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer 8:329–340. https://doi.org/10.1038/nrc2375
Soeth E, Grigoleit U, Moellmann B, Röder C, Schniewind B, Kremer B, Kalthoff H, Vogel I (2005) Detection of tumor cell dissemination in pancreatic ductal carcinoma patients by CK 20 RT-PCR indicates poor survival. J Cancer Res Clin Oncol 131:669–676
Su D, Yamaguchi K, Tanaka M (2005) The characteristics of disseminated tumor cells in pancreatic cancer: a black box needs to be explored. Pancreatology 5:316–324
Katz MH, Hwang R, Fleming JB, Evans DB (2008) Tumor-node-metastasis staging of pancreatic adenocarcinoma. CA Cancer J Clin 58:111–125. https://doi.org/10.3322/CA.2007.0012
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Farkouh A, Ettlinger D, Schueller J, Georgopoulos A, Scheithauer W, Czejka M (2010) A rapid and simple HPLC assay for quantification of capecitabine for drug monitoring purposes. Anticancer Res 30:5207–5211
Buchner P, Mihola E, Sahmanovic A, Steininger T, Dittrich C, Czejka M (2013) Validation of a simple assay for the quantification of the capecitabine metabolites 5′-DFCR and 5′-DFUR for drug monitoring in patients receiving outpatient chemotherapy. Anticancer Res 33:881–886
Lepper ER, Swain SM, Tan AR, Figg WD, Sparreboom A (2003) Liquid-chromatographic determination of erlotinib (OSI-774), an epidermal growth factor receptor tyrosine kinase inhibitor. J Chromatogr B Anal Technol Biomed Life Sci 796:181–188
Brennan B, Siu L, Dhesy-Thind B, Cripps C, Gandhi A, Abt M, Smith K, Rittweger K, Hussain S, Choudhury S (2007) Pharmacokinetic (PK) interactions between capecitabine (X), oxaliplatin (O) and bevacizumab (A) when used in combination for first-line treatment of metastatic colorectal cancer (MCRC). J Clin Oncol 25(suppl):S110. https://doi.org/10.1200/jco.2007.25.18_suppl.2554. (abstract 2554)
Herbst RS, Johnson DH, Mininberg E, Carbone DP, Henderson T, Kim ES, Blumenschein G Jr, Lee JJ, Liu DD, Truong MT, Hong WK, Tran H, Tsao A, Xie D, Ramies DA, Mass R, Seshagiri S, Eberhard DA, Kelley SK, Sandler A (2005) Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J Clin Oncol 23:2544–2555
Königsberg R, Gneist M, Jahn-Kuch D, Pfeiler G, Hager G, Hudec M, Dittrich C, Zeillinger R (2010) Circulating tumor cells in metastatic colorectal cancer: efficacy and feasibility of different enrichment methods. Cancer Lett 293:117–123. https://doi.org/10.1016/j.canlet.2010.01.003
Borgen E, Naume B, Nesland JM, Kvalheim G, Beiske K, Fodstad O, Diel I, Solomayer EF, Theocharous P, Coombes RC, Smith BM, Wunder E, Marolleau JP, Garcia J, Pantel K (1999) Standardization of the immunocytochemical detection of cancer cells in BM and blood: I. Establishment of objective criteria for the evaluation of immunostained cells. Cytotherapy 1:377–388. https://doi.org/10.1080/0032472031000141283
Tempero MA, Berlin J, Ducreux M, Haller D, Harper P, Khayat D, Schmoll HJ, Sobrero A, Van Cutsem E (2011) Pancreatic cancer treatment and research: an international expert panel discussion. Ann Oncol 22:1500–1506. https://doi.org/10.1093/annonc/mdq545
Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K, Shimamura T, Sho M, Kitano M, Cheng AL, Mizumoto K, Chen JS, Furuse J, Funakoshi A, Hatori T, Yamaguchi T, Egawa S, Sato A, Ohashi Y, Okusaka T, Tanaka M (2013) Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 31:1640–1648. https://doi.org/10.1200/JCO.2012.43.3680
Manji GA, Olive KP, Saenger YM, Oberstein P (2017) Current and emerging therapies in metastatic pancreatic cancer. Clin Cancer Res 23:1670–1678. https://doi.org/10.1158/1078-0432.CCR-16-2319
Kulke MH, Blaszkowsky LS, Ryan DP, Clark JW, Meyerhardt JA, Zhu AX, Enzinger PC, Kwak EL, Muzikansky A, Lawrence C, Fuchs CS (2007) Capecitabine plus erlotinib in gemcitabine-refractory advanced pancreatic cancer. J Clin Oncol 25:4787–4792
Ko AH, Venook AP, Bergsland EK, Kelley RK, Korn WM, Dito E, Schillinger B, Scott J, Hwang J, Tempero MA (2010) A phase II study of bevacizumab plus erlotinib for gemcitabine-refractory metastatic pancreatic cancer. Cancer Chemother Pharmacol 66:1051–1057. https://doi.org/10.1007/s00280-010-1257-5
Ko AH, Youssoufian H, Gurtler J, Dicke K, Kayaleh O, Lenz HJ, Keaton M, Katz T, Ballal S, Rowinsky EK (2012) A phase II randomized study of cetuximab and bevacizumab alone or in combination with gemcitabine as first-line therapy for metastatic pancreatic adenocarcinoma. Investig New Drugs 30:1597–1606. https://doi.org/10.1007/s10637-011-9691-8
Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, Verslype C, Scheithauer W, Shang A, Cosaert J, Moore MJ (2009) Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol 27:2231–2237. https://doi.org/10.1200/JCO.2008.20.0238
Reni M, Cereda S, Milella M, Novarino A, Passardi A, Mambrini A, Di Lucca G, Aprile G, Belli C, Danova M, Bergamo F, Franceschi E, Fugazza C, Ceraulo D, Villa E (2013) Maintenance sunitinib or observation in metastatic pancreatic adenocarcinoma: a phase II randomised trial. Eur J Cancer 49:3609–3615. https://doi.org/10.1016/j.ejca.2013.06.041
Starling N, Watkins D, Cunningham D, Thomas J, Webb J, Brown G, Thomas K, Oates J, Chau I (2009) Dose finding and early efficacy study of gemcitabine plus capecitabine in combination with bevacizumab plus erlotinib in advanced pancreatic cancer. J Clin Oncol 27:5499–5505. https://doi.org/10.1200/JCO.2008.21.5384
Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O'Reilly E, Wozniak TF, Picus J, Bhargava P, Mayer RJ, Schilsky RL, Goldberg RM (2010) Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 28:3617–3622. https://doi.org/10.1200/JCO.2010.28.1386
Chadha AS, Skinner HD, Gunther JR, Munsell MF, Das P, Minsky BD, Delclos ME, Chatterjee D, Wang H, Clemons M, George G, Singh PK, Katz MH, Fleming JB, Javle MM, Wolff RA, Varadhachary GR, Crane CH, Krishnan S (2016) Phase I trial of consolidative radiotherapy with concurrent bevacizumab, erlotinib and capecitabine for unresectable pancreatic cancer. PLoS One 11:e0156910. https://doi.org/10.1371/journal.pone.0156910. Published online 23 June 2016
Kindler HL, Ioka T, Richel DJ, Bennouna J, Létourneau R, Okusaka T, Funakoshi A, Furuse J, Park YS, Ohkawa S, Springett GM, Wasan HS, Trask PC, Bycott P, Ricart AD, Kim S, Van Cutsem E (2011) Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol 12:256–262. https://doi.org/10.1016/S1470-2045(11)70004-3
Rougier P, Riess H, Manges R, Karasek P, Humblet Y, Barone C, Santoro A, Assadourian S, Hatteville L, Philip PA (2013) Randomised, placebo-controlled, double-blind, parallel-group phase III study evaluating aflibercept in patients receiving first-line treatment with gemcitabine for metastatic pancreatic cancer. Eur J Cancer 49:2633–2642. https://doi.org/10.1016/j.ejca.2013.04.002
Lambrechts D, Claes B, Delmar P, Reumers J, Mazzone M, Yesilyurt BT, Devlieger R, Verslype C, Tejpar S, Wildiers H, de Haas S, Carmeliet P, Scherer SJ, Van Cutsem E (2012) VEGF pathway genetic variants as biomarkers of treatment outcome with bevacizumab: an analysis of data from the AViTA and AVOREN randomised trials. Lancet Oncol 13:724–733. https://doi.org/10.1016/S1470-2045(12)70231-0
Han K, Peyret T, Marchand M, Quartino A, Gosselin NH, Girish S, Allison DE, Jin J (2016) Population pharmacokinetics of bevacizumab in cancer patients with external validation. Cancer Chemother Pharmacol 78:341–351. https://doi.org/10.1007/s00280-016-3079-6
Farkouh A, Scheithauer W, Buchner P, Georgopoulos A, Schueller J, Gruenberger B, Czejka M (2014) Clinical pharmacokinetics of capecitabine and its metabolites in combination with the monoclonal antibody bevacizumab. Anticancer Res 34:3669–3673
Gil-Delgado M, Bastian G, Spano J, Paule B, Des-Guetz G, Bardier-Dupas A, Khayat D (2009) Oxaliplatin/capecitabine combination (Xelox) with or without targeted therapies in advanced colorectal cancer (ACRC) and pharmacokinetic analysis. J Clin Oncol 27(suppl):abstract e15068. https://doi.org/10.1200/jco.2009.27.15s.e15068
Pronk LC, Vasey P, Sparreboom A, Reigner B, Planting AS, Gordon RJ, Osterwalder B, Verweij J, Twelves C (2000) A phase I and pharmacokinetic study of the combination of capecitabine and docetaxel in patients with advanced solid tumours. Br J Cancer 83:22–29
Louie SG, Ely B, Lenz HJ, Albain KS, Gotay C, Coleman D, Raghavan D, Shields AF, Gold PJ, Blanke CD (2013) Higher capecitabine AUC in elderly patients with advanced colorectal cancer (SWOGS0030). Br J Cancer 109:1744–1749. https://doi.org/10.1038/bjc.2013.517
Twelves C, Trigo JM, Jones R, De Rosa F, Rakhit A, Fettner S, Wright T, Baselga J (2008) Erlotinib in combination with capecitabine and docetaxel in patients with metastatic breast cancer: a dose-escalation study. Eur J Cancer 44:419–426. https://doi.org/10.1016/j.ejca.2007.12.011
Ma WW, Herman JM, Jimeno A, Laheru D, Messersmith WA, Wolfgang CL, Cameron JL, Pawlik TM, Donehower RC, Rudek MA, Hidalgo M (2010) A tolerability and pharmacokinetic study of adjuvant erlotinib and capecitabine with concurrent radiation in resected pancreatic cancer. Transl Oncol 3:373–379
Van Cutsem E, Verslype C, Beale P, Clarke S, Bugat R, Rakhit A, Fettner SH, Brennscheidt U, Feyereislova A, Delord JP (2008) A phase Ib dose-escalation study of erlotinib, capecitabine and oxaliplatin in metastatic colorectal cancer patients. Ann Oncol 19:332–339
Petit-Jean E, Buclin T, Guidi M, Quoix E, Gourieux B, Decosterd LA, Gairard-Dory AC, Ubeaud-Séquier G, Widmer N (2015) Erlotinib: another candidate for the therapeutic drug monitoring of targeted therapy of cancer? A pharmacokinetic and pharmacodynamic systematic review of literature. Ther Drug Monit 37:2–21. https://doi.org/10.1097/FTD.0000000000000097
Bidard FC, Ferrand FR, Huguet F, Hammel P, Louvet C, Malka D, Boige V, Ducreux M, Andre T, de Gramont A, Mariani P, Pierga JY (2012) Disseminated and circulating tumor cells in gastrointestinal oncology. Crit Rev Oncol Hematol 82:103–115. https://doi.org/10.1016/j.critrevonc.2011.05.008
Bidard FC, Huguet F, Louvet C, Mineur L, Bouché O, Chibaudel B, Artru P, Desseigne F, Bachet JB, Mathiot C, Pierga JY, Hammel P (2013) Circulating tumor cells in locally advanced pancreatic adenocarcinoma: the ancillary CirCe 07 study to the LAP 07 trial. Ann Oncol 24:2057–2061. https://doi.org/10.1093/annonc/mdt176
Tjensvoll K, Nordgård O, Smaaland R (2014) Circulating tumor cells in pancreatic cancer patients: methods of detection and clinical implications. Int J Cancer 134:1–8. https://doi.org/10.1002/ijc.28134
de Albuquerque A, Kubisch I, Breier G, Stamminger G, Fersis N, Eichler A, Kaul S, Stölzel U (2012) Multimarker gene analysis of circulating tumor cells in pancreatic cancer patients: a feasibility study. Oncology 82:3–10. https://doi.org/10.1159/000335479
Han L, Chen W, Zhao Q (2014) Prognostic value of circulating tumor cells in patients with pancreatic cancer: a meta-analysis. Tumour Biol 35:2473–2480. https://doi.org/10.1007/s13277-013-1327-5
Ma XL, Li YY, Zhang J, Huang JW, Jia HY, Liu L, Li P (2014) Prognostic role of circulating tumor cells in patients with pancreatic cancer: a meta-analysis. Asian Pac J Cancer Prev 15:6015–6020
Acknowledgements
The authors would like to acknowledge the valuable contributions of Maria Lichtneckert and Guenther Nirnberger to the process of data management.
This trial was funded by the Central European Anticancer Drug Development Platform (CEADDP) via a grant (number 811590) of the Austrian Research Promotion Agency (FFG) in the frame of its SELP (Strategisches Exzellenz Leitprojekt) Structural Programme with added support from Roche Austria, the sponsor of the study (Roche Protocol # ML 20784).
Funding
This trial was funded by the Central European Anticancer Drug Development Platform (CEADDP) via a grant (number 811590) of the Austrian Research Promotion Agency (FFG) in the frame of its SELP (Strategisches Exzellenz Leitprojekt) Structural Programme with added support from Roche Austria, the sponsor of the study (Roche Protocol # ML 20784). Roche Austria was integrated into the design of the study, the collection of data, and the review of the manuscript. The company did not influence the production of the manuscript nor the data analysis nor the interpretation of the data presented.
Author information
Authors and Affiliations
Contributions
Conception and design: CD, RK, JP-D, MC, PB.
Acquisition of data: CD, RK, MM, KG, AS-H, MC, PB.
Analysis and interpretation of data: MM, CD, PB, RK, MC.
Writing (CD, MM, PB), review and/or revision of the manuscript: all authors.
Approval of the final version: all authors.
Agreed to be responsible and accountable for the results presented: all authors.
Corresponding author
Ethics declarations
Conflict of interest
CD’s non-profit research institutes (Ludwig Boltzmann-Institute for Applied Cancer Research (LBI-ACR VIEnna) and Applied Cancer Research – Institution for Translational Research Vienna, both forming the Central European Anticancer Drug Development Platform (CEADDP)) were funded by the Austrian Research Promotion Agency (FFG) – SELP (Strategisches Exzellenz Leitprojekt) Structural Programme, and the LBI-ACR VIEnna additionally by Roche Austria. CD received compensation as a member of a scientific advisory board of Roche Austria. He also consulted for Roche Austria and received compensation.
MM has received compensation for statistical analyses of the study from Roche Austria.
KG has received compensation as a member of scientific advisory boards and for presentations from Roche Austria.
JP-D is Roche Austria employee.
RK, AS-H, MC, and PB declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. The study protocol was approved by the Ethics Committee of the City of Vienna (reference number EK 08-159-0908).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
ESM 1
(DOCX 65 kb)
Rights and permissions
About this article
Cite this article
Dittrich, C., Königsberg, R., Mittlböck, M. et al. Phase Ib trial combining capecitabine, erlotinib and bevacizumab in pancreatic adenocarcinoma - REBECA trial. Invest New Drugs 37, 127–138 (2019). https://doi.org/10.1007/s10637-018-0639-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0639-0