Summary
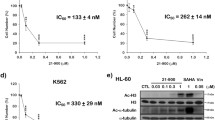
A panel of novel ellipticine isomers were designed and synthesised with the aim of evaluating their anti-cancer effects on selected leukaemia cell lines. A preliminary NCI 60-cell screen demonstrated that these compounds display promising anti-tumour activity across a number of different cell types. We have consequently examined the effect of these derivatives in detail on the Acute Myeloid Leukaemia (AML) cell line, MV4-11. Cell cycle analyses revealed that the compounds had a range of distinctive cell cycle effects. 7-Hydroxyisoellipticine showed the most promise with respect to cytostatic activity. We demonstrated that this compound inhibited proliferation of leukaemia cells by preventing cells from progressing from G2 phase into mitosis over a period of 24 h at a concentration of 5 μM. Our research suggests that this is mediated by an induction of reactive oxygen species (ROS), which in turn activates the DNA damage response pathway. As a result of the activation of p53, cyclin B1 is inhibited. The induction of this pathway leads to apoptosis which is seen at 48 h using the same dose of 7-hydroxyisoellipticine. This study provides for the first time detailed cellular information on the potential use of isoellipticines as chemotherapeutic agents.








Similar content being viewed by others
References
Goodwin S, Smith AF, Horning EC (1959) Alkaloids of ochrosia elliptica labill. J Am Chem Soc 81:1903–1908
Dalton LK, Demerac S, Elmes BC, Loder JW, Swan JM, Teitei T (1967) Synthesis of the tumor-inhibitory alkaloids, ellipticine, 9-methoxyellipticine, and related pyrido-[4,3-b]carbazoles. Aust J Chem 20:2715–2727
Sbai M, Lyazidi SA, Lerner DA, Castillo DB, Martin MA (1996) Stoichiometry and association constants of the inclusion complexes of ellipticine with modified P-cyclodextrin. Analyst 121:1561–1564
Miller CM, O’Sullivan EC, Devine KJ, McCarthy FO (2012) Synthesis and biological evaluation of novel isoellipticine derivatives and salts. Org Biomol Chem 10(39):7912–7921
Preisler HD, Lyman GH (1977) Am J of Haematol 3(3):209–218
Handin RI, Lux SE, Stosse TP, Babior BM (2003) Blood: principles and practice of hematology, 2nd edition, Lippincott. Williams and Wilkins, Philidelphia, pp 483–530
Lowenberg B (1996) Treatment of the elderly patient with acute myeloid leukaemia. Baillieres Clin Haematol 9(1):147–159
O’ Sullivan EC, Miller CM, Deane FM, McCarthy FO (2012) Emerging targets in the bioactivity of ellipticines and derivatives. Studies in natural products chemistry, chapter 6. Elsevier Science Publishers, Amsterdam, pp 189–226
Deane FM, O’Sullivan EC, Maguire AR, Gilbert J, Sakoff JA, McCluskey A et al (2013) Synthesis and evaluation of novel ellipticines as potential anti-cancer agents. Org Biomol Chem 11(8):1334–1344
Lerman LS (1961) Structural considerations in the interaction of DNA and acridines. J Mol Biol 3:18–30
Ross WE, Glaubiger D, Kohn KW (1978) Protein-associated DNA breaks in cells treated with Adriamycin or ellipticine. Biochim Biophys Acta 519(1):23–30
Auclair C, Paoletti C (1981) Bioactivation of the antitumor drugs 9-hydroellipticine and derivatives by a peroxidase-hydrogen peroxide system. J Med Chem 24(3):289–295
Kuo PL, Hsu YL, Kuo YC, Chang CH, Lin CC (2005) The anti-proliferative inhibition of ellipticine in human breast mda-mb-231 cancer cells is through cell cycle arrest and apoptosis induction. Anticancer Drugs 7:789–795
Hagg M, Berndtsson M, Mandic A, Zhou R, Shoshan MC, Linder S (2004) Induction of endoplasmic reticulum stress by ellipticine plant alkaloids. Mol Cancer Ther 3:489–497
Jin X, Gossett DR, Wang S, Yang D, Cao Y, Chen J et al (2004) Inhibition of AKT survival pathway by a small molecule inhibitor in human endometrial cancer cells. Br J Cancer 91(10):1808–1812
Vendome J, Letard S, Martin F, Svinarchuk F, Dubreuil P, Auclair C et al (2005) Molecular modelling of wild-type and D816V c-kit inhibition based on ATP-competitive binding of ellipticine derivatives to tyrosine kinases. J Med Chem 48:6194–6201
Prudent R, Moucadel V, Nguyen CH, Barette C, Schmidt F, Florent JC, Lafanechere L, Sautel CF, Duchemin-Pelletier E, Spreux E, Filhol O, Reiser JB, Cochet C (2010) Antitumor activity of pyridocarbazole and benzopyridoindole derivatives that inhibit protein kinase CK2. Cancer Res 70(23):9865–9874
Peng Y, Li C, Chen L, Sebti S, Chen J (2003) Rescue of Mutant p53 transcription function by ellipticine. Oncogene 22(29):4478–4487
Liu B, Chen Y, St Clair DK (2008) ROS and p53: a versatile partnership. Free Radic Biol Med 44(8):1529–1535
Chung YW, Jeong DW, Won JY, Choi EJ, Choi YH, Kim IJ (2002) H2O2-induced AP-1 activation and its effect on p21WAF1/CIP1-mediated G2/M arrest in a p53-deficient human lung cancer cell. Biochem Biophys Res Commun 293:1248–1253
Kreis NN, Sanhaji M, Rieger MA, Louwen F, Yuan J (2013) p21Waf1/Cip1 deficiency causes multiple mitotic defects in tumor cells. Oncogene. doi:10.1038/onc.2013.518
Simbulan-Rosenthal CM, Rosenthal DS, Iyer S, Boulares H, Smulson ME (1999) Involvement of PARP and poly(ADP-ribosyl)ation in the early stages of apoptosis and DNA replication. Mol Cell Biochem 193:137–148
Gribble GW, Saulnier MG, Obaza-Nutaitis JA, Ketcha DM (1992) J Org Chem 57:5891–5899
Kim JY, Lee SG, Chung JY, Kim YJ, Park JE, Koh H et al (2011) Ellipticine induces apoptosis in human endometrial cancer cells: the potential involvement of reactive oxygen species and mitogen-activated protein kinases. Toxicology 289(2–3):91–102
Owusu-Ansah E, Yavari A, Banerjee U (2008) A protocol for in vivo detection of reactive oxygen species. Protoco Exch. doi:10.1038/nprot.2008.23
Bennett MR (2001) Reactive oxygen species and death: oxidative DNA damage in atherosclerosis. Circ Res 88:648–650
Lakin ND, Jackson SP (1999) Regulation of p53 in response to DNA damage. Oncogene 18(53):7644–7655
Girardi C, James P, Zanin S, Pinna LA, Ruzzene M (2014) Differential phosphorylation of Akt1 and Akt2 by protein kinase CK2 may account for isoform specific functions. Biochim Biophys Acta 1843(9):1865–1874
Meggio F, Pinna LA (2003) One-thousand-and-one substrates of protein kinase CK2? FASEB J 17(3):349–368
Lee SR, Park JH, Park EK, Chung CH, Kang SS, Bang OS (2005) Akt-induced promotion of cell-cycle progression at G2/M phase involves upregulation of NF-Y binding activity in PC12 cells. J Cell Physiol 205(2):270–277
Gutierrez GJ, Tsuji T, Cross JV, Davis RJ, Templeton DJ, Jiang W, Ronai ZA (2010) JNK-mediated phosphorylation of Cdc25C regulates cell cycle entry and G2/M DNA damage checkpoint. J Biol Chem 285:14217–14228
Park EJ, Kiselev E, Conda-Sheridan M, Cushman M, Pezzuto JM (2012) Induction of apoptosis by 3-amino-6-(3-aminopropyl)-5,6-dihydro-5,11-dioxo-11H-indeno[1,2-c]isoquinoline via modulation of MAPKs (p38 and c-Jun N-terminal kinase) and c-Myc in HL-60 human leukemia cells. J Nat Prod 75(3):378–384
Porter AG, Janicke RU (1999) Emerging roles of caspase 3 in apoptosis. Cell Death Differ 6(2):99
Sureau F, Moreau F, Millot JM, Manfait M, Allard B, Aubard J et al (1993) Microspectrofluorometry of the protonation state of ellipticine, an antitumor alkaloid, in single cells. Biophys J 65(5):1767–1774
Schumacker PT (2006) Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell 10(3):175–176
Thorn T, GniadeckiR PAB, Vicanova J, Wulf HC (2001) Differences in activation of G2/M checkpoint in keratinocytes after genotoxic stress induced by hydrogen peroxide and ultraviolet radiation. Free Radic Res 35:405–416
Zhang Z, Huang C, Li J, Leonard SS, Lanciotti R, Butterworth L et al (2001) Vanadate-induced cell growth regulation and the role of reactive oxygen species. Arch Biochem Biophys 392:311–320
Zhang Z, Leonard SS, Huang C, Vallyathan V, Castranova V, Shi X (2003) Role of reactive oxygen species and MAPKs in vanadate-induced G2/M phase arrest. Free Radic Biol Med 34:1333–1342
Bijur GN, Briggs B, Hitchcock CL, Williams MV (1999) Ascorbic acid-dehydroascorbate induces cell cycle arrest at G2/M DNA damage checkpoint during oxidative stress. Environ Mol Mutagen 33:144–152
Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A (2004) H2AX: the histone guardian of the genome. DNA Repair 3:959–967
Fragkos M, Jurvansuu J, Beard P (2009) H2AX is required for cell cycle arrest via the p53/p21 pathway. Mol Cell Biol 29(10):2828–2840
Sugikawa E, Hosoi T, Yazaki N, Gamanuma M, Nakanishi N, Ohashi M (1999) Mutant p53 mediated induction of cell cycle arrest and apoptosis at G1 phase by 9-hydroxyellipticine. Anticancer Res 19(4B):3099–3108
Ohashi M, Sugikawa E, Nakanishi N (1995) Inhibition of p53 protein phosphorylation by 9-hydroxyellipticine: a possible anticancer mechanism. Jpn J Cancer Res 86(9):819–827
Cohen GM (1997) Caspases: the executioners of apoptosis. Biochem J 326:1–16
Acknowledgments
This work was supported by the Programme for Research in Third-Level Institutions (PRTLI), the Irish Cancer Society and the Children’s Leukaemia Research Project and the Irish Research Council by means of an IRCSET scholarship award.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Russell, E.G., O’Sullivan, E.C., Miller, C.M. et al. Ellipticine derivative induces potent cytostatic effect in acute myeloid leukaemia cells. Invest New Drugs 32, 1113–1122 (2014). https://doi.org/10.1007/s10637-014-0140-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-014-0140-3