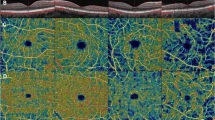
Abstract
Purpose
To compare and correlate retinal microcirculation and function in patients with non-proliferative diabetic retinopathy (NPDR).
Methods
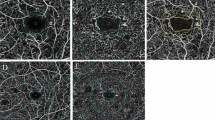
Thirty-three healthy controls (33 eyes), 36 diabetic patients with no clinically detectable retinopathy (NDR, 36 eyes) and 101 patients (101 eyes) with NPDR (35 mild NPDR, 34 moderate NPDR, 32 severe NPDR) were involved in the study. We used optical coherence tomography angiography (OCTA) to quantify the macular vessel density (VD) of superficial capillary plexus (SCP), deep capillary plexus (DCP) and foveal density in a 300 μm region around foveal avascular zone. Retinal function was assessed by a mydriasis-free, full-field flicker electroretinogram (FERG) recording device, and the amplitudes and implicit time were recorded. The association between microvascular parameters and FERG results was analyzed with stepwise multiple linear regression model.
Results
Decreased amplitudes and delayed implicit time, as well as lower parafoveal/perifoveal VD in both SCP and DCP, were found in NDR group and NPDR groups compared with the control group (all p < 0.05). Specifically, the FERG parameters and microvascular indices were comparable between NDR group and mild NPDR group (all p > 0.05). However, compared to mild NPDR, the reduction in FERG amplitude was more pronounced than the reduction in parafoveal VD (both SCP and DCP) in severe NPDR. Stepwise multiple linear regression analyses showed that delayed implicit time was significantly correlated with increased age and decreased VD of parafoveal region in both SCP and DCP in patients with NPDR. Meanwhile, decreased amplitude was significantly associated with decreased VD of parafoveal region in both SCP and DCP in patients with NPDR.
Conclusion
Macular VD in both superficial and deep capillary plexus correlated with ERG implicit time and amplitude in mild-to-severe NPDR. OCTA and FERG may both be useful in detection of preclinical DR and early DR, but once the disease deteriorates, FERG may be more sensitive to discern progression of DR.



Similar content being viewed by others
References
Yau JWY, Rogers S, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen S, Dekker JM, Fletcher AE, Grauslund J (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35(3):556–564
Ng JS, Bearse MA, Schneck ME, Barez S, Adams AJ (2008) Local diabetic retinopathy prediction by multifocal ERG delays over 3 years. Invest Ophthalmol Vis Sci 49(4):1622–1628
Harrison WW, Bearse MA, Ng JS, Jewell NP, Barez S, Burger D, Schneck ME, Adams AJ (2011) Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Invest Ophthalmol Vis Sci 52(2):772–777
Bearse MA, Han Y, Schneck ME, Barez S, Jacobsen C, Adams AJ (2004) Local multifocal oscillatory potential abnormalities in diabetes and early diabetic retinopathy. Invest Ophthalmol Vis Sci 45(9):3259–3265
Bearse MA, Adams AJ, Han Y, Schneck ME, Ng J, Bronson-Castain K, Barez S (2006) A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog Retin Eye Res 25(5):425–448. https://doi.org/10.1016/j.preteyeres.2006.07.001
Maa AY, Feuer WJ, Davis CQ, Pillow EK, Brown TD, Caywood RM, Chasan JE, Fransen SR (2016) A novel device for accurate and efficient testing for vision-threatening diabetic retinopathy. J Diabetes Complicat 30(3):524–532
Fukuo M, Hirose A, Kitano S, Kato K, Kondo M (2016) Screening for diabetic retinopathy using RETevalTM, new mydriasis-free full-field ERG recording system. Invest Ophthalmol Vis Sci 57(12):6340
Bresnick GH, Palta M (1987) Temporal aspects of the electroretinogram in diabetic retinopathy. Arch Ophthalmol 105(5):660–664
Santos AR, Ribeiro L, Bandello F, Lattanzio R, Egan C, Frydkjaer-Olsen U, García-Arumí J, Gibson J, Grauslund J, Harding SP, Lang GE, Massin P, Midena E, Scanlon P, Aldington SJ, Simão S, Schwartz C, Ponsati B, Porta M, Costa MÂ, Hernández C, Cunha-Vaz J, Simó R (2017) Functional and structural findings of neurodegeneration in early stages of diabetic retinopathy: cross-sectional analyses of baseline data of the EUROCONDOR project. Diabetes 66(9):2503–2510. https://doi.org/10.2337/db16-1453
Tyrberg M, Ponjavic V, Lovestamadrian M (2008) Multifocal electroretinogram (mfERG) in patients with diabetes mellitus and an enlarged foveal avascular zone (FAZ). Doc Ophthalmol 117(3):185–189
Jansson RW, Raeder MB, Krohn J (2015) Photopic full-field electroretinography and optical coherence tomography in type 1 diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 253(7):989–997
Sandhu HS, Eladawi N, Elmogy M, Keynton RS, Helmy O, Schaal S, Elbaz A (2018) Automated diabetic retinopathy detection using optical coherence tomography angiography: a pilot study. Br J Ophthalmol 102(11):1564–1569
Alam MN, Zhang Y, Lim JI, Chan RVP, Yang M, Yao X (2018) Quantitative optical coherence tomography angiography features for objective classification and staging of diabetic retinopathy. Retina. https://doi.org/10.1097/IAE.0000000000002373
American Diabetes Association (2014) Diagnosis and classification of diabetes mellitus. Diabetes Care 37(Supplement 1):S81
Wilkinson CP, Ferris FL, Klein R, Lee PP, Agardh C, Davis MD, Dills DG, Kampik A, Pararajasegaram R, Verdaguer J (2003) Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 110(9):1677–1682
Kato K, Kondo M, Sugimoto M, Ikesugi K, Matsubara H (2015) Effect of pupil size on flicker ERGs recorded With RETeval system: new mydriasis-free full-field ERG system. Invest Ophthalmol Vis Sci 56(6):3684–3690
Fukuo M, Kondo M, Hirose A, Fukushima H, Ikesugi K, Sugimoto M, Kato K, Uchigata Y, Kitano S (2016) Screening for diabetic retinopathy using new mydriasis-free, full-field flicker ERG recording device. Sci Rep 6(1):36591
Zeng Y, Cao D, Yu H, Yang D, Zhuang X, Hu Y, Li J, Yang J, Wu Q, Liu B, Zhang L (2019) Early retinal neurovascular impairment in patients with diabetes without clinically detectable retinopathy. Br J Ophthalmol. https://doi.org/10.1136/bjophthalmol-2018-313582
Campbell JP, Zhang M, Hwang TS, Bailey ST, Wilson DJ, Jia Y, Huang D (2017) Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci Rep 7:42201. https://doi.org/10.1038/srep42201
Arend O, Remky A, Evans D, Stuber R, Harris A (1997) Contrast sensitivity loss is coupled with capillary dropout in patients with diabetes. Invest Ophthalmol Vis Sci 38(9):1819–1824
Luu CD, Szental JA, Lee S, Lavanya R, Wong TY (2010) Correlation between retinal oscillatory potentials and retinal vascular caliber in type 2 diabetes. Invest Ophthalmol Vis Sci 51(1):482–486
Kondo M, Sieving PA (2001) Primate photopic sine-wave flicker ERG: vector modeling analysis of component origins using glutamate analogs. Invest Ophthalmol Vis Sci 42(1):305–312
Sapieha P (2012) Eyeing central neurons in vascular growth and reparative angiogenesis. Blood 120(11):2182–2194
Moran EP, Wang Z, Chen J, Sapieha P, Smith LEH, Ma J (2016) Neurovascular cross talk in diabetic retinopathy: pathophysiological roles and therapeutic implications. Am J Physiol Heart Circ Physiol 311(3):H738–H749
Durbin MK, An L, Shemonski N, Soares M, Santos T, Lopes MC, Neves C, Cunhavaz J (2017) Quantification of retinal microvascular density in optical coherence tomographic angiography images in diabetic retinopathy. JAMA Ophthalmol 135(4):370–376
Yonemura D, Tsuzuki K, Aoki T (1962) Clinical importance of the oscillatory potential in the human ERG. Acta Ophthalmol 40(S70):115–123. https://doi.org/10.1111/j.1755-3768.1962.tb00313.x
Tzekov R, Arden GB (1999) The electroretinogram in diabetic retinopathy. Surv Ophthalmol 44(1):53–60
Kooijman AC (1983) Light distribution on the retina of a wide-angle theoretical eye. J Opt Soc Am 73(11):1544–1550
Pflibsen KP, Pomerantzeff O, Ross RN (1988) Retinal illuminance using a wide-angle model of the eye. J Opt Soc Am A Opt Image Sci Vis 5(1):146–150
Bedell HE, Katz LM (1982) On the necessity of correcting peripheral target luminance for pupillary area. Optom Vis Sci 59(10):767–769
Funding
This study was supported by Grant 81800829 from National Natural Science Foundation of China to Cheng Yang and Grant 81870663 from National Natural Science Foundation of China to Honghua Yu. The sponsors or funding organizations had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Statement on the welfare of animals
No animals were used in this study.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeng, Y., Cao, D., Yang, D. et al. Retinal vasculature–function correlation in non-proliferative diabetic retinopathy. Doc Ophthalmol 140, 129–138 (2020). https://doi.org/10.1007/s10633-019-09724-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-019-09724-4