Abstract
Background
Poorly differentiated colorectal cancers are more aggressive. Metabolism reprogramming is a significant hallmark in cancer, and aerobic glycolysis is common. However, how cancer cells reprogramming glucose metabolism contributes to cell differentiation was largely unknown. Previous studies have reported that tumor suppressor NDRG2 could promote colorectal cancers differentiation.
Aims
This study aims to demonstrate that NDRG2 promotes the differentiation of colorectal cancers, potentially through the inhibition of aerobic glycolysis via TXNIP induction.
Methods
Western blotting, qRT-PCR and immunohistochemical staining were used to detect the expression of related molecules. MTT assay was used to reflect cell viability and proliferation. Immunofluorescent assay was performed to identify the expression and distribution of molecules. Luciferase analysis and CHIP assays were used to investigate the mechanism. Bioinformatic analysis was performed to predict the relevance.
Results
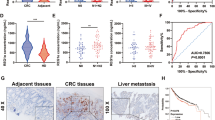
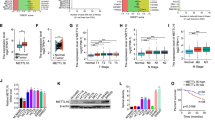
In colorectal cancers, NDRG2 could inhibit cell proliferation, reduce glucose uptake and decrease expression of key glycolysis enzymes. Upregulated NDRG2 is associated with differentiated cancer. However, deletion of TXNIP, a classic glucose metabolism inhibitor, could obviously alter the function of NDRG2 in differentiation, glucose uptake, expression of key glycolysis enzymes and proliferation. Mechanistically, high glucose flux promotes the activity of TXNIP promoter. And NDRG2 promotes the occupancy of transcription factor Mondo A on TXNIP promoter, predominantly through the suppression of c-myc, which could complete with Mondo A binding to TXNIP promoter. In clinical samples, high expression of TXNIP indicates good prognosis and outcome.
Conclusions
NDRG2-dependent induction of TXNIP is critical for the aerobic glycolysis during colorectal cancers differentiation.





Similar content being viewed by others
References
Brody H. Colorectal cancer. Nature 2015;521:S1.
Derwinger K, Kodeda K, Bexe-Lindskog E, Taflin H. Tumour differentiation grade is associated with TNM staging and the risk of node metastasis in colorectal cancer. Acta oncologica (Stockholm, Sweden) 2010;49:57–62.
Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell 2000;103:311–320.
Lorentzen A, Vogel LK, Lewinsky RH, Saebø M, Skjelbred CF, Godiksen S, Hoff G, Tveit KM, Lothe IM, Ikdahl T, Kure EH, Mitchelmore C. Expression of NDRG2 is down-regulated in high-risk adenomas and colorectal carcinoma. BMC cancer 2007;7:192.
Shen L, Qu X, Li H, Xu C, Wei M, Wang Q, Ru Y, Liu B, Xu Y, Li K, Hu J, Wang L, Ma Y, Li M, Lai X, Gao L, Wu K, Yao L, Zheng J, Zhang J. NDRG2 facilitates colorectal cancer differentiation through the regulation of Skp2-p21/p27 axis. Oncogene 2018;37:1759–1774.
Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell metabolism 2016;23:27–47.
Yoshida GJ. Metabolic reprogramming: the emerging concept and associated therapeutic strategies. Journal of experimental & clinical cancer research : CR 2015;34:111.
M. Peng, N. Yin, S. Chhangawala, K. Xu, C.S. Leslie, M.O. Li, Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism, Science (New York, N.Y.), 354 (2016) 481–484.
Almeida L, Lochner M, Berod L, Sparwasser T. Metabolic pathways in T cell activation and lineage differentiation. Seminars in immunology 2016;28:514–524.
Li M, Jia F, Zhou H, Di J, Yang M. Elevated aerobic glycolysis in renal tubular epithelial cells influences the proliferation and differentiation of podocytes and promotes renal interstitial fibrosis. European review for medical and pharmacological sciences 2018;22:5082–5090.
X. Zheng, L. Boyer, M. Jin, J. Mertens, Y. Kim, L. Ma, L. Ma, M. Hamm, F.H. Gage, T. Hunter, Metabolic reprogramming during neuronal differentiation from aerobic glycolysis to neuronal oxidative phosphorylation, eLife, 5 (2016).
Shen L, O’Shea JM, Kaadige MR, Cunha S, Wilde BR, Cohen AL, Welm AL, Ayer DE. Metabolic reprogramming in triple-negative breast cancer through Myc suppression of TXNIP. Proceedings of the National Academy of Sciences of the United States of America 2015;112:5425–5430.
Xu X, Li J, Sun X, Guo Y, Chu D, Wei L, Li X, Yang G, Liu X, Yao L, Zhang J, Shen L. Tumor suppressor NDRG2 inhibits glycolysis and glutaminolysis in colorectal cancer cells by repressing c-Myc expression. Oncotarget 2015;6:26161–26176.
Zhang J, Li F, Liu X, Shen L, Liu J, Su J, Zhang W, Deng Y, Wang L, Liu N, Han W, Zhang J, Ji S, Yang A, Han H, Yao L. The repression of human differentiation-related gene NDRG2 expression by Myc via Miz-1-dependent interaction with the NDRG2 core promoter. The Journal of biological chemistry 2006;281:39159–39168.
L. Shen, X. Qu, Y. Ma, J. Zheng, D. Chu, B. Liu, X. Li, M. Wang, C. Xu, N. Liu, L. Yao, J. Zhang, Tumor suppressor NDRG2 tips the balance of oncogenic TGF-β via EMT inhibition in colorectal cancer, Oncogenesis, 3 (2014) e86.
Wang S, Fan X, Zhu J, Xu D, Li R, Chen R, Hu J, Shen Y, Hao J, Wang K, Jiang X, Wang Y, Jiang Y, Li J, Zhang J. The differentiation of colorectal cancer is closely relevant to m6A modification. Biochemical and biophysical research communications 2021;546:65–73.
Abu El Maaty MA, Alborzinia H, Khan SJ, Büttner M, Wölfl S. 1,25(OH)(2)D(3) disrupts glucose metabolism in prostate cancer cells leading to a truncation of the TCA cycle and inhibition of TXNIP expression, Biochimica et biophysica acta. Molecular cell research 1864;2017:1618–1630.
Stoltzman CA, Peterson CW, Breen KT, Muoio DM, Billin AN, Ayer DE. Glucose sensing by MondoA: Mlx complexes: a role for hexokinases and direct regulation of thioredoxin-interacting protein expression. Proceedings of the National Academy of Sciences of the United States of America 2008;105:6912–6917.
Billin AN, Eilers AL, Coulter KL, Logan JS, Ayer DE. MondoA, a novel basic helix-loop-helix-leucine zipper transcriptional activator that constitutes a positive branch of a max-like network. Mol Cell Biol 2000;20:8845–8854.
Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities, Clinical cancer research : an official journal of the American Association for. Cancer Research 2009;15:6479–6483.
Kim YJ, Kang HB, Yim HS, Kim JH, Kim JW. NDRG2 positively regulates E-cadherin expression and prolongs overall survival in colon cancer patients. Oncology reports 2013;30:1890–1898.
Chu D, Zhang Z, Li Y, Wu L, Zhang J, Wang W, Zhang J. Prediction of colorectal cancer relapse and prognosis by tissue mRNA levels of NDRG2. Molecular cancer therapeutics 2011;10:47–56.
Y. Chen, J. Ning, W. Cao, S. Wang, T. Du, J. Jiang, X. Feng, B. Zhang, Research Progress of TXNIP as a Tumor Suppressor Gene Participating in the Metabolic Reprogramming and Oxidative Stress of Cancer Cells in Various Cancers, Frontiers in oncology, 10 (2020) 568574.
Kaadige MR, Looper RE, Kamalanaadhan S, Ayer DE. Glutamine-dependent anapleurosis dictates glucose uptake and cell growth by regulating MondoA transcriptional activity. Proceedings of the National Academy of Sciences of the United States of America 2009;106:14878–14883.
Hui ST, Andres AM, Miller AK, Spann NJ, Potter DW, Post NM, Chen AZ, Sachithanantham S, Jung DY, Kim JK, Davis RA. Txnip balances metabolic and growth signaling via PTEN disulfide reduction. Proceedings of the National Academy of Sciences of the United States of America 2008;105:3921–3926.
Zhu G, Zhou L, Liu H, Shan Y, Zhang X. MicroRNA-224 Promotes Pancreatic Cancer Cell Proliferation and Migration by Targeting the TXNIP-Mediated HIF1α Pathway. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 2018;48:1735–1746.
Gunes A, Iscan E, Topel H, Avci ST, Gumustekin M, Erdal E, Atabey N. Heparin treatment increases thioredoxin interacting protein expression in hepatocellular carcinoma cells. The international journal of biochemistry & cell biology 2015;65:169–181.
Chen D, Dang BL, Huang JZ, Chen M, Wu D, Xu ML, Li R, Yan GR. MiR-373 drives the epithelial-to-mesenchymal transition and metastasis via the miR-373-TXNIP-HIF1α-TWIST signaling axis in breast cancer. Oncotarget 2015;6:32701–32712.
Peterson CW, Stoltzman CA, Sighinolfi MP, Han KS, Ayer DE. Glucose controls nuclear accumulation, promoter binding, and transcriptional activity of the MondoA-Mlx heterodimer. Molecular and cellular biology 2010;30:2887–2895.
Miller DM, Thomas SD, Islam A, Muench D, Sedoris K. c-Myc and cancer metabolism, Clinical cancer research : an official journal of the American Association for. Cancer Research 2012;18:5546–5553.
Acknowledgments
This work was financially sponsored by grants from the National Natural Science Foundation of China (No. 82073053, No.81702845, No.81502370), the Key Research and Development Plan in Shaanxi Province of China (2020SF-081), the Natural Science Basic Research Plan in Shaanxi Province of China (2019JQ-879), the State Key Laboratory of Cancer Biology Project (CBSKL2019ZZ12; CBSKL2019KF03).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Hu, J., Feng, L., Ren, M. et al. Colorectal Cancer Cell Differentiation Is Dependent on the Repression of Aerobic Glycolysis by NDRG2-TXNIP Axis. Dig Dis Sci 67, 3763–3772 (2022). https://doi.org/10.1007/s10620-021-07188-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-07188-8