Abstract
Background
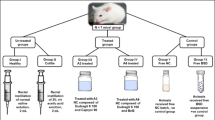
New formulations for topical treatment of ulcerative colitis with budesonide inclusion complex (BUDHP-β-CD) and poloxamers (PL) were developed for future clinical use.
Aims
This study evaluated the efficacy of such novel formulations in a rat model of colitis.
Methods
The PL-BUDHP-β-CD systems were prepared by direct dispersion of the complex (BUD concentration 0.5 mg mL−1) in solutions with PL407 or PL403. Male Wistar rats underwent TNBS-induced colitis and were treated for 5 days by a rectal route, as follows: BUD 1: BUDHP-β-CD + PL407 (18%); BUD 2: BUDHP-β-CD + PL407 (20%); BUD 3: BUDHP-β-CD + PL407 (18%) + PL403 (2%); BUD 4: plain BUD; BUD 5: BUDHP-β-CD; C1: HP-β-CD + PL407 (18%); C2: HP-β-CD + PL407 (20%); C3: HP-β-CD + PL407 (18%) + PL403 (2%); C4: saline. A negative control group without colitis was also used. Colitis was assessed via myeloperoxidase (MPO) activity, and macroscopic and microscopic damage score in colon tissues. Protein levels of TNF-α, IL-1β, IL-10 and endogenous glucocorticoids were obtained using ELISA.
Results
BUDHP-β-CD poloxamer formulations had similar MPO activity when compared with the negative control group. All formulations presented lower MPO activity than BUDHP-β-CD and plain BUD (p < 0.001). BUD 2 produced lower microscopic score values than plain BUD and BUDHP-β-CD (p < 0.01). All formulations with BUDHP-β-CD poloxamers reduced TNF-α levels (p < 0.05).
Conclusion
Novel budesonide inclusion complex formulations improved microscopic damage and reduced colonic MPO activity and TNF-α levels.






Similar content being viewed by others
References
Witaicenis A, Luchini AC, Hiruma-Lima CA, et al. Suppression of TNBS-induced colitis in rats by 4-methylesculetin, a natural coumarin: comparison with prednisolone and sulphasalazine. Chem Biol Interact. 2012;195:76–85.
Nyuyki KD, Pittman QJ. Toward a better understanding of the central consequences of intestinal inflammation. Ann N Y Acad Sci. 2015;1351:149–154.
Zeeff SB, Kunne C, Bouma G, de Vries RB, Te Velde AA. Actual usage and quality of experimental colitis models in preclinical efficacy testing: a scoping review. Inflamm Bowel Dis. 2016;22:1296–1305.
Leitner GC, Vogelsang H. Pharmacological- and non-pharmacological therapeutic approaches in inflammatory bowel disease in adults. World J Gastrointest Pharmacol Ther. 2016;7:5–20.
Christophi GP, Rengarajan A, Ciorba MA. Rectal budesonide and mesalamine formulations in active ulcerative proctosigmoiditis: efficacy, tolerance, and treatment approach. Clin Exp Gastroenterol. 2016;9:125–130.
Seibold F, Fournier N, Beglinger C, et al. Topical therapy is underused in patients with ulcerative colitis. J Crohns Colitis. 2014;8:56–63.
Iborra M, Alvarez-Sotomayor D, Nos P. Long-term safety and efficacy of budesonide in the treatment of ulcerative colitis. Clin Exp Gastroenterol. 2014;7:39–46.
Lichtenstein GR. Budesonide multi-matrix for the treatment of patients with ulcerative colitis. Dig Dis Sci. 2016;61:9.
Brunner M, Vogelsang H, Greinwald R, et al. Colonic spread and serum pharmacokinetics of budesonide foam in patients with mildly to moderately active ulcerative colitis. Aliment Pharmacol Ther. 2005;22:463–470.
Lemann M, Galian A, Rutgeerts P, et al. Comparison of budesonide and 5-aminosalicylic acid enemas in active distal ulcerative colitis. Aliment Pharmacol Ther. 1995;9:557–562.
Hartmann F, Stein J, BudMesa-Study Group. Clinical trial: controlled, open, randomized multicentre study comparing the effects of treatment on quality of life, safety and efficacy of budesonide or mesalazine enemas in active left-sided ulcerative colitis. Aliment Pharmacol Ther. 2010;32:368–376.
Rashid M, Kaur V, Hallan SS, Sharma S, Mishra N. Microparticles as controlled drug delivery carrier for the treatment of ulcerative colitis: a brief review. Saudi Pharm J. 2016;24:458–472.
Klouda L. Thermoresponsive hydrogels in biomedical applications: a seven-year update. Eur J Pharm Biopharm. 2015;97:338–349.
Papini JZB, Cereda CMS, Pedrazzoli Júnior J, Alafatti SA, de Araújo DR, Tofoli GR. Pharmacokinetics and pharmacodynamics evaluation of tramadol in thermoreversible gels. Biomed Res Int. 2017;2017:5954629.
Akkari AC, Ramos Campos EV, Keppler AF, et al. Budesonide–hydroxypropyl-β-cyclodextrin inclusion complex in binary poloxamer 407/403 system for ulcerative colitis treatment: a physico-chemical study from micelles to hydrogels. Colloids Surf B Biointerfaces. 2016;138:138–147.
Gotardo ÉM, Ribeiro Gde A, Clemente TR, et al. Hepcidin expression in colon during trinitrobenzene sulfonic acid-induced colitis in rats. World J Gastroenterol. 2014;20:4345–4352.
dos Reis SB, de Oliveira CC, Acedo SC, et al. Attenuation of colitis injury in rats using Garcinia cambogia extract. Phytother Res.. 2009;23:324–329.
Zar JH. Biostatistical Analysis. 5th ed. Prentice Hall: Upper Saddle River; 2010.
Randhawa PK, Singh K, Singh N, Jaggi AS. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol. 2014;18:279–288.
Ergang P, Leden P, Bryndová J, et al. Glucocorticoid availability in colonic inflammation of rat. Dig Dis Sci. 2008;53:2160–2167.
Coccia M, Harrison OJ, Schiering C, et al. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J Exp Med. 2012;209:1595–1609.
Li L, Liu Z, Yang X, Yan H, Bao S, Fei J. Bioluminescence imaging for IL-1β expression in experimental colitis. J Inflamm (Lond). 2013;10:16.
Acknowledgments
The authors are grateful to FAPESP (#2014/26200-9) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lázaro, C.M., de Oliveira, C.C., Gambero, A. et al. Evaluation of Budesonide–Hydroxypropyl-β-Cyclodextrin Inclusion Complex in Thermoreversible Gels for Ulcerative Colitis. Dig Dis Sci 65, 3297–3304 (2020). https://doi.org/10.1007/s10620-020-06075-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06075-y