Abstract
Background and Aims
Sodium-glucose cotransporter-2 (SGLT2) inhibitors are a novel class of drugs that lower glucose by inducing renal glycosuria. We aimed to explore whether SGLT2 inhibitor added to the usual care for patients with type 2 diabetes mellitus (T2DM) and biopsy-proven nonalcoholic steatohepatitis (NASH) will benefit NASH histology.
Methods
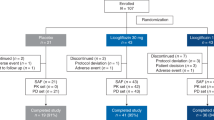
In this investigator-initiated, single-arm, open-label, pilot study, nine biopsy-proven NASH patients with T2DM were given empagliflozin 25 mg daily for 24 weeks. Liver biopsy was repeated at the end of treatment. The histological outcomes were compared with the placebo group of a previous 48-week clinical trial.
Results
There was a significant reduction in body mass index (median change, Δ = −0.7 kg per m2, p = 0.011), waist circumference (Δ = −3 cm, p = 0.033), systolic blood pressure (Δ = −9 mmHg, p = 0.024), diastolic blood pressure (Δ = −6 mmHg, p = 0.033), fasting blood glucose (Δ = −1.7 mmol/L, p = 0.008), total cholesterol (Δ = −0.5 mmol/L, p = 0.011), gamma glutamyl transpeptidase (Δ = −19 U/L, p = 0.013), volumetric liver fat fraction (Δ = −7.8%, p = 0.017), steatosis (Δ = −1, p = 0.014), ballooning (Δ = −1, p = 0.034), and fibrosis (Δ = 0, p = 0.046). All histological components either remained unchanged or improved, except in one patient who had worsening ballooning. Empagliflozin resulted in significantly greater improvements in steatosis (67% vs. 26%, p = 0.025), ballooning (78% vs. 34%, p = 0.024), and fibrosis (44% vs. 6%, p = 0.008) compared with historical placebo.
Conclusion
This pilot study provides primary histological evidence that empagliflozin may be useful for the treatment of NASH. This preliminary finding should prompt larger clinical trials to assess the effectiveness of empagliflozin and other SGLT2 inhibitors for the treatment of NASH in T2DM patients.
Trial registry number ClincialTrials.gov number, NCT02964715.



Similar content being viewed by others
References
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84.
Chan WK, Tan AT, Vethakkan SR, Tah PC, Vijayananthan A, Goh KL. Non-alcoholic fatty liver disease in diabetics—prevalence and predictive factors in a multiracial hospital clinic population in Malaysia. J Gastroenterol Hepatol. 2013;28:1375–1383.
Cusi K. Treatment of patients with type 2 diabetes and non-alcoholic fatty liver disease: current approaches and future directions. Diabetologia. 2016;59:1112–1120.
Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649.
Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555.
Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690.
Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med. 2010;27:136–142.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128.
Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care. 2016;39:1115–1122.
Abdul-Ghani M, Del Prato S, Chilton R, DeFronzo RA. SGLT2 inhibitors and cardiovascular risk: lessons learned from the EMPA-REG OUTCOME study. Diabetes Care. 2016;39:717–725.
Chan WK, Nik Mustapha NR, Mahadeva S. Controlled attenuation parameter for the detection and quantification of hepatic steatosis in nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2014;29:1470–1476.
Yoneda M, Yoneda M, Fujita K, et al. Transient elastography in patients with non-alcoholic fatty liver disease (NAFLD). Gut. 2007;56:1330–1331.
Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023.
Anuurad E, Shiwaku K, Nogi A, et al. The new BMI criteria for asians by the regional office for the western pacific region of WHO are suitable for screening of overweight to prevent metabolic syndrome in elder Japanese workers. J Occup Health. 2003;45:335–343.
Alberti KG, Zimmet P, Shaw J, Group IDFETFC. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366:1059–1062.
Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94:709–728.
Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321.
St Pierre TG, House MJ, Bangma SJ, et al. Stereological analysis of liver biopsy histology sections as a reference standard for validating non-invasive liver fat fraction measurements by MRI. PLoS ONE. 2016;11:e0160789.
Ratziu V, Harrison SA, Francque S, et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150:1147e1145–1159e1145.
Wah Kheong C, Nik Mustapha NR, Mahadeva S. A randomized trial of silymarin for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2017;15:1940e1948–1949e1948.
Tahara A, Kurosaki E, Yokono M, et al. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol. 2013;715:246–255.
Qiang S, Nakatsu Y, Seno Y, et al. Treatment with the SGLT2 inhibitor luseogliflozin improves nonalcoholic steatohepatitis in a rodent model with diabetes mellitus. Diabetol Metab Syndr. 2015;7:104.
Tang L, Wu Y, Tian M, et al. Dapagliflozin slows the progression of the renal and liver fibrosis associated with type 2 diabetes. Am J Physiol Endocrinol Metab. 2017;313:E563–E576.
Jojima T, Tomotsune T, Iijima T, Akimoto K, Suzuki K, Aso Y. Empagliflozin (an SGLT2 inhibitor), alone or in combination with linagliptin (a DPP-4 inhibitor), prevents steatohepatitis in a novel mouse model of non-alcoholic steatohepatitis and diabetes. Diabetol Metab Syndr. 2016;8:45.
Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “Thrifty Substrate” hypothesis. Diabetes Care. 2016;39:1108–1114.
Lee PCH, Gu Y, Yeung MY, et al. Dapagliflozin and empagliflozin ameliorate hepatic dysfunction among chinese subjects with diabetes in part through glycemic improvement: a single-center, retrospective, observational study. Diabetes Ther. 2018;9:285–295.
Seko Y, Sumida Y, Sasaki K, et al. Effects of canagliflozin, an SGLT2 inhibitor, on hepatic function in Japanese patients with type 2 diabetes mellitus: pooled and subgroup analyses of clinical trials. J Gastroenterol. 2018;53:140–151.
Leiter LA, Forst T, Polidori D, Balis DA, Xie J, Sha S. Effect of canagliflozin on liver function tests in patients with type 2 diabetes. Diabetes Metab. 2016;42:25–32.
Charatcharoenwitthaya P, Lindor KD, Angulo P. The spontaneous course of liver enzymes and its correlation in nonalcoholic fatty liver disease. Dig Dis Sci. 2012;57:1925–1931.
Wong VW, Wong GL, Tsang SW, et al. Metabolic and histological features of non-alcoholic fatty liver disease patients with different serum alanine aminotransferase levels. Aliment Pharmacol Ther. 2009;29:387–396.
Takeda A, Irahara A, Nakano A, et al. The improvement of the hepatic histological findings in a patient with non-alcoholic steatohepatitis with type 2 diabetes after the administration of the sodium-glucose cotransporter 2 inhibitor ipragliflozin. Intern Med. 2017;56:2739–2744.
Akuta N, Watanabe C, Kawamura Y, et al. Effects of a sodium-glucose cotransporter 2 inhibitor in nonalcoholic fatty liver disease complicated by diabetes mellitus: Preliminary prospective study based on serial liver biopsies. Hepatol Commun. 2017;1:46–52.
Kuchay MS, Krishan S, Mishra SK, et al. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT trial). Diabetes Care. 2018;41:1801–1808.
Acknowledgment
The authors would like to thank Wan Nur Illyana Wan Yusoff, Wan Noor Hidayu, Talvant Kaur, and Soudamany Nair, for their assistance in the research project.
Funding
This study was funded by the University of Malaya Special Research Fund (Project No.: BK006-2016 and BKS067-2017). University of Malaya was not involved in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
The histological outcomes of patients who received empagliflozin 25 mg daily for 24 weeks compared with patients with type 2 diabetes mellitus from the placebo group of a 48-week clinical trial previously conducted at the same center (TIFF 1374 kb)
Rights and permissions
About this article
Cite this article
Lai, LL., Vethakkan, S.R., Nik Mustapha, N.R. et al. Empagliflozin for the Treatment of Nonalcoholic Steatohepatitis in Patients with Type 2 Diabetes Mellitus. Dig Dis Sci 65, 623–631 (2020). https://doi.org/10.1007/s10620-019-5477-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-5477-1