Abstract
Aims
Inhibitor for the apoptosis-stimulating protein of p53 (iASPP) has been reported to be correlated with 5-fluorouracil (5-Fu) resistance in renal cell carcinoma. Here, we uncover mechanisms of iASPP-Nrf2-ROS regulation of 5-Fu resistance which are important for the development of alternative treatment strategies for gastric adenocarcinoma treatment.
Methods
We analyzed iASPP and Nrf2 through TCGA RNA-seq data, UALCAN analysis, and cBioPortal datasets. Intracellular ROS generation was determined by 2′,7′-dichloro-fluorescin diacetate staining. Transwell was used to evaluate the invasion. The expression of iASPP, Nrf2, HO-1, and GSTP1 was tested using western blot.
Results
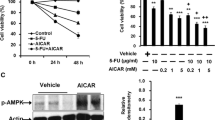
We found that iASPP KD led to an apparent 5-Fu-induced ROS accumulation in MGC803 and SCG790 cells. Accompanied by iASPP KD, Nrf2 was markedly decreased. iASPP-induced ROS inhibition relies on Nrf2, and due to both knocked down iASPP and Nrf2, the level of ROS did not show an obvious difference with Nrf2 KD solely. Similarly, iASPP KD failed to enhance the Nrf2 KD-mediated ROS accumulation after 5-Fu treatment, suggesting that iASPP-induced antioxidative effects related to 5-Fu resistance are partially dependent on Nrf2. Also, the combination of iASPP KD and Nrf2 KD did not show any synergistic effect on apoptosis after 5-Fu treatment in MGC803 and SCG790 cells. Further studies revealed that iASPP KD or Nrf2 KD could decrease the expression of HO-1 and GSTP1.
Conclusions
Our data highlight that iASPP plays a crucial role in the inhibition of 5-Fu-induced apoptosis resistance by removing ROS accumulation in gastric adenocarcinoma, and that the removal of ROS induced by iASPP is Nrf2 signaling dependent.





Similar content being viewed by others
References
Maleki SS, Rocken C. Chromosomal instability in gastric cancer biology. Neoplasia. 2017;19:412–420.
Nishikawa S, Konno M, Hamabe A, et al. Aldehyde dehydrogenase high gastric cancer stem cells are resistant to chemotherapy. Int J Oncol. 2013;42:1437–1442.
Yoon C, Park DJ, Schmidt B, et al. CD44 expression denotes a subpopulation of gastric cancer cells in which Hedgehog signaling promotes chemotherapy resistance. Clin Cancer Res. 2014;20:3974–3988.
Guo X, Ma N, Wang J, et al. Increased p38-MAPK is responsible for chemotherapy resistance in human gastric cancer cells. BMC Cancer. 2008;8:375.
Zhou D, Liu W, Liang S, et al. Apoptin-derived peptide reverses cisplatin resistance in gastric cancer through the PI3K-AKT signaling pathway. Cancer Med. 2018;7:1369–1383.
Ye XL, Zhao YR, Weng GB, et al. IL-33-induced JNK pathway activation confers gastric cancer chemotherapy resistance. Oncol Rep. 2015;33:2746–2752.
Zheng P, Chen L, Yuan X, et al. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36:53.
Ge W, Zhao K, Wang X, et al. iASPP is an antioxidative factor and drives cancer growth and drug resistance by competing with Nrf2 for Keap1 binding. Cancer Cell. 2017;32:561–573.e6.
Zhou Z, Song J, Nie L, Chen X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem Soc Rev. 2016;45:6597–6626.
Mansingh DP, Sunanda OJ, Sali VK, Vasanthi HR. [6]-Gingerol-induced cell cycle arrest, reactive oxygen species generation, and disruption of mitochondrial membrane potential are associated with apoptosis in human gastric cancer (AGS) cells. J Biochem Mol Toxicol. 2018;32:e22206.
Hou XS, Wang HS, Mugaka BP, Yang GJ, Ding Y. Mitochondria: promising organelle targets for cancer diagnosis and treatment. Biomater Sci. 2018;6:2786–2797.
Tamaki R, Kanai-Mori A, Morishige Y, Koike A, Yanagihara K, Amano F. Effects of 5-fluorouracil, adriamycin and irinotecan on HSC-39, a human scirrhous gastric cancer cell line. Oncol Rep. 2017;37:2366–2374.
Fu L, Yin F, Li XR, et al. Generation and characterization of a paclitaxel-resistant human gastric carcinoma cell line. Anticancer Drugs. 2018;29:491–502.
Wu Q, Yu JC, Liu YQ, Kang WM, Guo WD. Effect of combination of docosahexaenoic acid and fluorouracil on human gastric carcinoma cell strain MGC803. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2010;32:65–70.
Jiang L, Siu MK, Wong OG, et al. iASPP and chemoresistance in ovarian cancers: effects on paclitaxel-mediated mitotic catastrophe. Clin Cancer Res. 2011;17:6924–6933.
Liu ZJ, Cai Y, Hou L, et al. Effect of RNA interference of iASPP on the apoptosis in MCF-7 breast cancer cells. Cancer Invest. 2008;26:878–882.
Qiu S, Liu S, Yu T, et al. Sertad1 antagonizes iASPP function by hindering its entrance into nuclei to interact with P53 in leukemic cells. BMC Cancer. 2017;17:795.
Yang JP, Hori M, Takahashi N, Kawabe T, Kato H, Okamoto T. NF-kappaB subunit p65 binds to 53BP2 and inhibits cell death induced by 53BP2. Oncogene. 1999;18:5177–5186.
Lu X. p53: a heavily dictated dictator of life and death. Curr Opin Genet Dev. 2005;15:27–33.
Bergamaschi D, Samuels Y, O’Neil NJ, et al. iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human. Nat Genet. 2003;33:162–167.
Notari M, Hu Y, Koch S, et al. Inhibitor of apoptosis-stimulating protein of p53 (iASPP) prevents senescence and is required for epithelial stratification. Proc Natl Acad Sci U S A. 2011;108:16645–16650.
Wang Y, Xu SL, Wu YZ, et al. Simvastatin induces caspase-dependent apoptosis and activates P53 in OCM-1 cells. Exp Eye Res. 2013;113:128–134.
Hayes JD, Dinkova-Kostova AT. Oncogene-stimulated congestion at the KEAP1 stress signaling hub allows bypass of NRF2 and induction of NRF2-target genes that promote tumor survival. Cancer Cell. 2017;32:539–541.
Chan KK, Wong OG, Wong ES, et al. Impact of iASPP on chemoresistance through PLK1 and autophagy in ovarian clear cell carcinoma. Int J Cancer. 2018;143:1456–1469.
Kurtishi A, Rosen B, Patil KS, Alves GW, Moller SG. Cellular proteostasis in neurodegeneration. Mol Neurobiol. 2018;56:3676–3689.
He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem. 2017;44:532–553.
Saha S, Bhattacharjee P, Mukherjee S, et al. Contribution of the ROS-p53 feedback loop in thuja-induced apoptosis of mammary epithelial carcinoma cells. Oncol Rep. 2014;31:1589–1598.
Chen T, Deng Z, Zhao R, Shen H, Li W. SYKT alleviates doxorubicin-induced cardiotoxicity via modulating ROS-mediated p53 and MAPK signal pathways. Evid Based Complement Altern Med. 2018;2018:2581031.
Yang Y, Zhu Y, Xi X. Anti-inflammatory and antitumor action of hydrogen via reactive oxygen species. Oncol Lett. 2018;16:2771–2776.
Satyavarapu EM, Das R, Mandal C, Mukhopadhyay A, Mandal C. Autophagy-independent induction of LC3B through oxidative stress reveals its non-canonical role in anoikis of ovarian cancer cells. Cell Death Dis. 2018;9:934.
Weinberg F, Chandel NS. Reactive oxygen species-dependent signaling regulates cancer. Cell Mol Life Sci. 2009;66:3663–3673.
Weinberg F, Hamanaka R, Wheaton WW, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107:8788–8793.
Wallace DC. Mitochondria and cancer. Nat Rev Cancer. 2012;12:685–698.
Moon DO, Kim MO, Choi YH, Hyun JW, Chang WY, Kim GY. Butein induces G(2)/M phase arrest and apoptosis in human hepatoma cancer cells through ROS generation. Cancer Lett. 2010;288:204–213.
Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12:931–947.
Prasad S, Gupta SC, Tyagi AK. Reactive oxygen species (ROS) and cancer: role of antioxidative nutraceuticals. Cancer Lett. 2017;387:95–105.
Liu J, Wang Z. Increased oxidative stress as a selective anticancer therapy. Oxid Med Cell Longev. 2015;2015:294303.
Sodrul IMD, Wang C, Chen X, Du J, Sun H. Role of ginsenosides in reactive oxygen species-mediated anticancer therapy. Oncotarget. 2018;9:2931–2950.
Xie ZZ, Liu Y, Bian JS. Hydrogen sulfide and cellular redox homeostasis. Oxid Med Cell Longev. 2016;2016:6043038.
Ahmad I, Shukla S, Singh D, et al. CYP2E1-mediated oxidative stress regulates HO-1 and GST expression in maneb-and paraquat-treated rat polymorphonuclear leukocytes. Mol Cell Biochem. 2014;393:209–222.
Bolduc JA, Collins JA, Loeser RF. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic Biol Med. 2018;132:73–82.
Trang NT, Huyen VT, Tuan NT, Phan TD. Association of N-acetyltransferase-2 and glutathione S-transferase polymorphisms with idiopathic male infertility in Vietnam male subjects. Chem Biol Interact. 2018;286:11–16.
Zheng L, Jiang WD, Feng L, et al. Selenium deficiency impaired structural integrity of the head kidney, spleen and skin in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2018;82:408–420.
Alqarni MH, Muharram MM, Labrou NE. Ligand-induced glutathione transferase degradation as a therapeutic modality: investigation of a new metal-mediated affinity cleavage strategy for human GSTP1-1. Int J Biol Macromol. 2018;116:84–90.
Acknowledgments
This work was supported by funds from the National Natural Science Foundation of China (81602721).
Author information
Authors and Affiliations
Contributions
LW, HF, and JH contributed to conception and design. SY, PL, and RC developed the methodology. LW contributed to acquisition of data. LW, SY, and QW contributed to analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis). LW, BK, and JH wrote, reviewed, and/or revised the manuscript. HF contributed to administrative, technical, or material support (i.e., reporting or organizing data, constructing databases).
Corresponding author
Ethics declarations
Conflict of interest
The author reports no conflicts of interest in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wen, L., Yang, S., Li, P. et al. iASPP-Mediated ROS Inhibition Drives 5-Fu Resistance Dependent on Nrf2 Antioxidative Signaling Pathway in Gastric Adenocarcinoma. Dig Dis Sci 65, 2873–2883 (2020). https://doi.org/10.1007/s10620-019-06022-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-06022-6