Abstract
In cases of difficult biliary cannulation, transpancreatic sphincterotomy (TPS) can be an alternative approach of biliary access. However, its success and safety profile have not been studied in detail. A systematic review and meta-analysis were performed to study the overall cannulation success and adverse events of TPS. These outcomes were also compared to other advanced cannulation methods. A systematic literature search was conducted to find all relevant articles containing data on TPS. Successful biliary cannulation and complications rates [post-ERCP pancreatitis (PEP), bleeding, and perforation rates] were compared in the pooled analyses of prospective comparative studies. The overall outcomes were calculated involving all studies on TPS. TPS was superior compared to needle-knife precut papillotomy (NKPP) and the double-guidewire method (DGW) regarding cannulation success (odds ratio [OR] 2.32; 95% confidence interval [CI] 1.37–3.93; and OR 2.72; 95% CI 1.30–5.69, respectively). The rate of PEP did not differ between TPS and NKPP or DGW; however, TPS (only retrospective studies were available for comparison) proved to be worse than needle-knife fistulotomy in this regard (OR 4.62; 95% CI 1.36–15.72). Bleeding and perforation rates were similar among these advanced techniques. There were no data about long-term consequences of TPS. The biliary cannulation rate of TPS is higher than that of the other advanced cannulation techniques, while the safety profile is similar to those. However, no long-term follow-up studies are available on the later consequences of TPS; therefore, such studies are strongly needed for its full evaluation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biliary access during endoscopic retrograde cholangiopancreatography (ERCP) is successful after a few attempts with basic cannulation methods in around 80% of the cases. The European Society of Gastrointestinal Endoscopy (ESGE) recommends guidewire-assisted cannulation over contrast material injection during the initial attempts because of the higher rate of success and a lower rate of post-ERCP pancreatitis (PEP) [1]. However, in challenging cases, the initial attempts to achieve selective biliary cannulation can fail even in the hands of experienced endoscopists. A consensus definition of difficult biliary cannulation is still lacking. The current ESGE guideline defines it as more than five contacts with the papilla while attempting to cannulate, more than 5 min spent attempting to cannulate the papilla after visualization, or more than one unintended pancreatic duct cannulation or opacification. The time limit of the standard cannulation technique is extended to 10 min, but other aspects are identical in another new international recommendation [2]. Early use of advanced cannulation techniques is advised in these situations to prevent further papillary trauma. Two scenarios are possible in case of failed biliary access: Needle-knife precut methods or pancreatic guidewire-assisted methods can be applied if the guidewire is inserted into the pancreatic duct [1].
Pancreatic guidewire-assisted methods can be classified as single-guidewire methods (cannulation attempts, contrast material injection, or precut after leaving the guidewire in the pancreatic duct), double-guidewire technique (DGW) [3], and transpancreatic (biliary) sphincterotomy (TPS) [4]. A recent meta-analysis of randomized controlled trials showed that the DGW technique has a higher PEP rate compared to other advanced methods despite its relative “noninvasiveness” [5]. Our previous meta-analysis showed that TPS is an effective technique which provides a higher rate of successful biliary access; furthermore, its application results in lower bleeding and PEP rates when compared to needle-knife precut papillotomy (NKPP) [6].
The needle-knife precut techniques are freehand precut starting either from the papillary orifice (NKPP) or at the papillary roof (needle-knife fistulotomy, NKF). These techniques can also be applied after pancreatic guidewire or prophylactic pancreatic stents (PPS) insertion. In fact, some studies are showing better outcomes (i.e., higher success and lower complication rates) with this method compared to the freehand precut [7]. NKPP with a small incision over a pancreatic stent improves the success rate and reduces the complication rate in difficult biliary cannulations [7] or when compared to standard cannulation [8]. Some studies suggest that NKF is superior to NKPP in terms of success and complications, providing a lower PEP rate by avoiding the trauma of the orifice [9].
In the present systematic review, the efficacy and safety of the rarely used TPS technique are scrutinized further by comparing them with other frequently used advanced cannulation methods. TPS was first described by Goff et al. [4], and he published results from 51 patients with remarkable success rate and safety profile of TPS later on [10]. Since then, several case series, retrospective and prospective comparative studies, and few randomized controlled trials (RCTs) have been published. On the other hand, concerns have been raised about the long-term safety of this technique [11]. The possibility of pancreatic stenosis, as seen in the cases of therapeutic pancreatic sphincterotomies, should not be ignored [11, 12]. Here, we summarize the available evidence of the success rate, immediate, and late adverse events related to TPS in comparison with other advanced cannulation methods by executing a systematic review.
Methods
Search Strategy
A systematic literature search was conducted to find all relevant articles containing data on TPS in accordance with the PRISMA guideline [13]. The search strategy included the following terms: “transpancreatic septotomy” or “transpancreatic sphincterotomy” or “transpancreatic septostomy” or “transpancreatic precut sphincterotomy” or “pancreatic sphincterotomy” or “transpancreatic papillary septotomy” or “transpancreatic sphincter precut” or “transpancreatic duct precut” or “pancreatic sphincter precutting” or “pancreatic precut sphincterotomy” or “transpancreatic precut septotomy” or “transpancreatic precut septostomy” or “pancreatic septotomy” or “pancreatic septostomy” or “pancreatic precut” or “transpancreatic precut” or “transpancreatic.” EMBASE, PubMed, Scopus, Web of Science, ProQuest, and Cochrane Library databases were searched from their inception till February 8, 2018.
Inclusion Criteria
In order to compare TPS to DGW and NKPP, only prospective studies were included. However, only retrospective data were available in the comparison of TPS–NKF, and these were also included in our analysis. Appropriate conference abstracts were also analyzed to minimize publication bias, and additional subgroup analyses excluding them were carried out to show their effects on outcomes.
Comparative and also non-comparative prospective and retrospective studies were included in the calculation of overall success and complications rate of TPS. Randomized controlled trials (RCT) and prospective and retrospective observational studies were analyzed separately (Table 4).
Study Selection and Data Collection
Titles and abstracts of studies identified were screened by two authors (D.P. and Á.V.) independently, and then, the full-text articles were searched to identify eligible studies. Data extraction and risk of bias assessment were done independently by the authors. Peer-reviewed works and conference abstracts were included. Unpublished data were not requested from the authors. Any disagreement was resolved by discussion in plenum. Prophylactic measures to prevent PEP; furthermore, the length and results of follow-up were also collected and analyzed.
Risk of Bias Assessment
The Newcastle–Ottawa scale (NOS) was used for prospective and retrospective studies to assess risk of bias within the individual studies [14] (Table 5). Randomized controlled trials were assessed by the Cochrane Risk of Bias Tool [15] (Table 6).
Statistical Methods
Pooled odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated to compare the biliary cannulation success and PEP rates among the different cannulation techniques. Risk difference (RD) was calculated to compare the bleeding and perforation rates in order to avoid overestimation since OR or RR calculations would exclude those studies where zero events were reported. The random-effect model of DerSimonian and Laird [16] was used in meta-analysis. Subgroup analyses excluding studies with sequential designs and that reported only in an abstract format were also carried out. Sensitivity analyses were carried out using four types of summary statistics (RR [risk ratio] vs. OR vs. RD vs. Peto’s OR) and two types of meta-analytical models (fixed vs. random effects) to test the robustness of our findings [17]. Heterogeneity was tested with two methods, namely the Cochrane’s Q and the I2 statistics. The Q test was computed by summing the squared deviations of each study’s estimate from the overall meta-analysis estimate; P values were obtained by comparing the statistical results with a χ2 distribution with k − 1 degrees of freedom (where k was the number of studies). A P value of less than 0.1 was considered suggestive of significant heterogeneity. The I2 statistic represents the percentage of the total variability across studies that is due to heterogeneity, i.e., I2 value between 0 and 40% indicates low, 30–60% moderate, 50–90% substantial, and 75–100% considerable heterogeneity, based on Cochrane Handbook for Systematic Reviews of Interventions [17]. Publication bias was planned to be examined by visual inspection of funnel plots and the Egger’s method [18]. Meta-analytical calculations were done with Review Manager (RevMan) computer program (version 5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Results
Study Selection
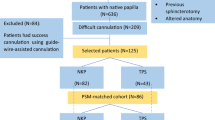
Altogether, 2787 records were identified during database search: 510 in EMBASE, 339 in PubMed, 968 in Scopus, 255 in Web of Science, 544 in ProQuest, and 171 in Cochrane Library, respectively. The latest search was run on February 8, 2018, and finally, 33 relevant studies were included in the qualitative synthesis, while data from 14 studies were extracted for the meta-analysis (Fig. 1).
Characteristics of Studies Included
Characteristics of the included studies with the applied PEP prophylaxis (Table 1), the definitions of difficult biliary access and the endoscopists/centers experience (Table 2), and the late adverse events are summarized in Table 3.
Three RCTs [19,20,21] and two prospective observational studies [22, 23] compared TPS and DGW. One of them was only available in abstract form [19]. Two of them used a sequential design [22, 23], applying TPS only after DGW, as a rescue technique.
Two RCTs [24, 25] and three prospective observational studies [22, 23, 26] provided data on the comparison of TPS vs. NKPP, two of them with sequential design [22, 23]. New prospective studies were not identified after our previous meta-analysis; however, we conducted further sensitivity and subgroup analyses in this comparison [6].
Comparison of TPS and NKF was not found in any prospective studies; four retrospective studies (two of them only in abstract form) were identified and analyzed to synthesize available comparative evidence [9, 27,28,29].
Two prospective case series of TPS without relevant comparisons to other advanced cannulation methods [30, 31] and, additionally, 23 retrospective observational studies with reported outcome data were included in the pooled analyses of overall outcomes of TPS [4, 9, 10, 27,28,29, 32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48] (Table 4).
Methodological Quality and Risk of Bias Assessment
The risk of bias in the prospective (not RCTs) and the four retrospective studies included in the meta-analyses was analyzed with the NOS (Table 5). In most of the full-text studies, baseline characteristics of cohorts were reported with comparable, homogeneous groups. Technical details of interventions were thoroughly reported; all full-text studies defined precut methods appropriately. On the other hand, definitions of adverse outcomes somewhat varied; however, most studies used the consensus definitions [49]. The appropriate length of follow-up is questionable in the cases of late adverse events, and only one prospective study reported the length of follow-up as longer than 30 days [30]. The abstracts contained limited information about the above-mentioned details; therefore, they carry an unclear risk of bias.
In case of RCTs, the Cochrane Risk of Bias Tool was used (Table 6). Only one study [21] reported the method of randomization and the method of ensuring allocation concealment. Blinding in studies of endoscopic interventions at participant and personnel level is difficult to execute and therefore could not be expected. However, blinded late outcome assessment (PEP, late bleeding, perforation) could be arranged more easily. Nevertheless, none of the studies reported blinding of any kind. Three out of five RCTs did not report the rate of cholangitis; therefore, this outcome could not be analyzed [19, 24, 25]. One RCT was only published in abstract form which makes the data quality questionable; consequently, this study carries a high risk of bias [19].
Publication bias could not be reliably assessed based on funnel plots or by the Egger’s method because of the small number of included studies. According to the Cochrane Handbook, funnel plots and other statistical tests are not advised to assess small study effect and publication bias under ten studies per analysis [17, 18, 50].
Endoscopists’ Experience and Centers’ Case Volumes in the Prospective Studies
Most of the prospective studies reported endoscopists’ experience in yearly case numbers, some in lifetime ERCP numbers, too. Based on the reported numbers, all endoscopists performed more than 200 ERCPs/year. In one study, the caseload of the endoscopists exceeded 500 ERCPs annually [30]. Trainee participation was not reported in any of the studies. Most of the centers reported high-volume ERCPs (even above 1000 procedures/year [23, 24]), only one study [9] reported lower numbers (< 300 ERCPs/year), while no information was found about center or endoscopist caseload in one study [29] (Table 2).
Biliary Cannulation Success Rate
TPS showed superiority in success rate compared to DGW (OR 2.72; 95% CI 1.30–5.69; 176 and 235 patients, respectively; I2 = 50%) (Fig. 2a) and NKPP (OR 2.32; 95% CI 1.37–3.93; 292 and 260 patients, respectively; I2 = 7%) (Fig. 2b). The success rate of TPS and NKF did not differ (OR 1.38; 95% CI 0.32–5.96; 295 and 141 patients, respectively; I2 = 22%) (Fig. 2c).
a Forest plot of cannulation success rate of transpancreatic sphincterotomy (TPS) versus double-guidewire technique (DGW) in prospective studies; b comparison of cannulation success rate of TPS versus needle-knife precut papillotomy (NKPP) in prospective studies; c comparison of cannulation success rate of TPS versus needle-knife fistulotomy (NKF) in available comparative retrospective studies
In the TPS versus DGW comparison of cannulation success rates, no significant difference was detected between the two methods if only RCTs were included (OR 3.02; 95% CI 0.73–12.59; 113 and 107 patients, respectively; I2 = 69%), probably because of the greater confidence intervals of the results. On the other hand, subgroup analysis of full-text studies found the superiority of TPS over DGW with regard to cannulation success rate (Suppl. Figure 1).
The overall success rate of TPS in prospective studies was 89.7% (564/629). The success rate was the same if all studies were analyzed (89.6%, 2343/2615), as well as the separate analysis of RCTs resulted in similarly high value (91.7%, 199/217) (Table 4).
Post-ERCP Pancreatitis
No significant difference was found between the TPS versus DGW (OR 0.72; 95% CI 0.24–2.10; 151 and 134 patients, respectively; I2 = 55%) (Fig. 3a) and TPS versus NKPP (OR 1.63; 95% CI 0.48–5.47; 265 and 242 patients, respectively; I2 = 57%) (Fig. 3b) comparisons. However, the TPS technique showed a higher PEP rate compared to NKF method (OR 4.62; 95% CI 1.36–15.72; 295 and 141 patients, respectively; I2 = 16%) (Fig. 3c).
a Forest plot of post-ERCP pancreatitis (PEP) rate of transpancreatic sphincterotomy (TPS) versus double-guidewire technique (DGW) in prospective studies; b comparison of PEP rate of TPS versus needle-knife precut papillotomy (NKPP) in prospective studies; c comparison of PEP rate of TPS versus needle-knife fistulotomy (NKF) in available comparative retrospective studies
If we excluded abstracts from the NKF versus TPS comparison, the significant difference disappeared (OR 3.49; 95% CI 0.20–62.21; 86 and 115 patients, respectively; I2 = 63%) and expectedly, a wide confidence interval could be seen (Suppl. Figure 2). In the other subgroups, no differences were found when sequential studies or abstracts were omitted from the analyses. Inclusion of RCTs only did not result any change in significance regarding TPS versus DGW and TPS versus NKPP comparisons.
The overall PEP rate of TPS was 8.1% (49/604) in prospective studies, 7.1% (183/2590) in all studies, and 7.4% (16/217) in RCTs (Table 4).
Prophylactic Pancreatic Stent and Nonsteroid Anti-inflammatory Suppository Use
Only one recently published study used PPS in all patients undergoing TPS [20], while all the others reported no or only some PPS implantation in the TPS cases (Table 1). Pharmacologic prevention of PEP was applied in three studies [20, 27, 28]; however, the recommended nonsteroid anti-inflammatory drug (NSAID) suppositories were not used or not reported in any of the studies included in the meta-analyses (Table 1).
Bleeding
The pooled analysis did not show any difference in bleeding rate when TPS was compared to DGW (risk difference [RD] 0.01; 95% CI − 0.03 to 0.05; 109 and 95 patients, respectively; I2 = 0%) (Fig. 4a), NKPP (RD − 0.00; 95% CI − 0.04 to 0.03; 268 and 239 patients, respectively; I2 = 20%) (Fig. 4b), and NKF (RD 0.00; 95% CI − 0.03 to 0.03; 295 and 141 patients, respectively; I2 = 0%) (Fig. 4c).
a Forest plot of bleeding rate after transpancreatic sphincterotomy (TPS) versus double-guidewire technique (DGW) in prospective studies; b comparison of bleeding rate after TPS versus needle-knife precut papillotomy (NKPP) in prospective studies; c comparison of bleeding rate after TPS versus needle-knife fistulotomy (NKF) in available comparative retrospective studies
Subgroup analyses did not alter the findings of bleeding rates significantly.
The overall bleeding rate of TPS was 3.4% (19/562) in prospective studies, 2.0% (50/2548) in all studies, and 1.7% (3/175) in RCTs (Table 4).
Perforation
Perforation rates did not differ when comparing TPS versus DGW (RD − 0.01; 95% CI − 0.04 to 0.03; 109 vs. 95; I2 = 0%) (Fig. 5a), TPS versus NKPP (RD − 0.00; 95% CI − 0.02 to 0.01; 267 and 240 patients, respectively; I2 = 0%) (Fig. 5b), and TPS versus NKF (RD 0.00; 95% CI − 0.02 to 0.03; 295 and 141 patients, respectively; I2 = 0%) (Fig. 5c).
a Forest plot of comparison of perforation rate after transpancreatic sphincterotomy (TPS) versus double-guidewire technique (DGW) in prospective studies; b comparison of perforation rate after TPS versus needle-knife precut papillotomy (NKPP) in prospective studies; c comparison of perforation rate after TPS versus needle-knife fistulotomy (NKF) in available comparative retrospective studies
Subgroup analyses did not alter the findings in perforations rates significantly.
The overall perforation rate was 0.5% (3/562) in prospective studies, 0.4% (11/2548) in all studies, while 0% (0/175) in RCTs (Table 4).
Sensitivity and Subgroup Analyses
Application of other meta-analytical models (fixed-effect vs. random-effect analysis) and summary statistics (OR vs. RR vs. RD vs. Peto’s OR) did not affect the outcomes significantly in the main analyses; thus, our conclusions remain unaltered (Suppl. Table 1).
However, subgroup analyses excluding non-RCTs, sequential trials, and studies only available in an abstract form altered significantly some results (i.e., success rate in TPS vs. DGW and PEP rate in TPS vs. NKF comparisons, respectively) (Suppl. Table 2, Figs. 1 and 2).
Follow-Up
Pancreatic duct stricture or chronic pancreatitis could potentially develop after pancreatic sphincterotomy; therefore, a longer follow-up period is needed to detect these adverse outcomes [11]. Small caliber pancreatic stents could rarely cause pancreatic ductal changes in long term (1 month or longer) [51, 52]. Only one prospective study, a case series with 116 patients, reported a median 5-month follow-up (range 2–35) with no late adverse events [30]. Another paper similarly did not report late chronic pancreatitis or ductitis from PPS; no strictures were described during longer, however not specified, follow-up [22] (Table 3). A few retrospective studies also published longer-term results: Miao et al. [45] reported no stricture after 4 months of follow-up period, while Barakat et al. [33] found no late stricture formation after an unknown length of “long-term” follow-up.
Discussion
This systematic review and meta-analysis show that TPS could be equally successful or even slightly better in the setting of difficult biliary access compared to other advanced cannulation methods. Analyzing only the prospective studies with regard to cannulation success rates TPS seems superior to DGW and NKPP, while TPS and NKF are equally effective. DGW and NKPP carry a similar risk of PEP compared to TPS; however, PEP occurs more frequently with TPS than with NKF. No difference in bleeding and perforation rates was found when comparing TPS to the other advanced cannulation methods.
Prospective observational studies and RCTs were analyzed whenever it was possible to gain the best evidence. Between-study heterogeneity was low or moderate in most analyses, making our conclusions more accurate. Sensitivity analyses and application of different statistical and meta-analytical methods did not reveal any significant changes in the main associations. However, subgroup analyses excluding sequential studies revealed that the significant difference disappeared in some analyses, thereby weakening our conclusion in the findings of success rate of TPS versus DGW and PEP rate in TPS versus NKF. However, this is most probably the result of the low case numbers, leading to imprecision and wider confidence intervals.
Exceptionally low cannulation rates (as low as 72%) and high PEP rates (36.8%) were seen in the sequential studies (Table 4) that probably could be explained by the previous DGW attempts causing papillary trauma and consequential edema. Our experience also shows that TPS after papillary trauma induced by precut results low rate of biliary access, while it is highly successful if applied primarily [53]. Based on these considerations, we recommend the TPS technique in the early phase of difficult biliary access when pancreatic guidewire insertion reached unintentionally.
The overall cannulation success rate of TPS is close to 90% in all studies and also in subgroups by different study designs, which makes this pancreatic guidewire-assisted method a good alternative to DGW and other advanced cannulation methods. The overall biliary cannulation success rate of DGW was only 61% in the studies where it was compared to TPS (Fig. 2a). Furthermore, a meta-analysis of seven RCTs with DGW showed that successful biliary cannulation was achieved only in 82% of cases [5]. NKPP is also a frequently used method in cases of difficult biliary access. The average cannulation success rate of NKPP was approximately 80% (647/812) in all NKPP studies and 77% (201/260) in prospective studies according to our previous meta-analysis [6].
PEP rate of TPS is similar to other advanced cannulation methods (7.1%; 183/2590; 0–30%, Table 4). NKPP seems comparable to TPS with its 8.8% (70/794) overall PEP rate measured in our previous meta-analysis [6]. NKF, however, could be better to avoid PEP (Fig. 3c). With the uniform use of PPS and NSAID suppositories in all TPS cases, a PEP rate might be even lower [20, 48] as the significant protective effect of PPS has been well proven. Importantly, its insertion should not be problematic since the guidewire is already in the pancreatic duct while performing TPS.
Bleeding rate of TPS is in the range of 2–4%, which is comparable to the widely accepted and frequently used needle-knife precut techniques (4%; 30/745 of NKPP cases) [6]. The rate of perforation was around 0.5% which is remarkably low for a precut technique, and no difference was found in this respect between TPS and the other advanced cannulation techniques.
There are several limitations of our analyses. First of all, the low number of prospective studies with only small cohorts of patients weakens the conclusions. Sequential studies were also included which could alter our results. However, in the comparison of DGW or NKPP vs. TPS, sequential designs could affect the TPS cannulation success and adverse event rate only to the worse due to the prolonged cannulation attempt and greater trauma of the papilla. The lack of information on the use of effective preventive methods (PPS, NSAID suppositories) undermines the assessment of PEP rates. New studies are lacking in this field with the consistent use of PPS and NSAID suppositories. It should be noted, however, that the PEP rate was only 1.1% in the study of Sugiyama et al. [20], where all patients received PPS after TPS, compared to the rate of 7.1% pooled from all studies where most patients did not have PPS. Besides that, the definitions of outcomes were not standardized in all cases. Nonetheless, most prospective studies used the consensus definitions [49]. Publication bias cannot be ruled out due to the low number of studies per analysis.
The possible benefit of TPS over the freehand precut techniques is that it is a wire-assisted method, with better control of the cut. For that reason, it could be appealing in those situations, where the papillary tract is smaller, or the position of the scope is unstable. Furthermore, the PPS insertion could also be easily achieved after the precut, since the guidewire is already inserted into the pancreatic duct. An additional benefit is that the sphincterotome does not need to be changed for the precut. In the unfortunate cases, when TPS fails, additional needle-knife incision could be helpful to reach deep biliary cannulations and might be used as salvage technique in appropriate situations.
The late adverse events of TPS, e.g., pancreatic duct stricture and chronic pancreatitis [11], could not be assessed properly because only one prospective study reported a longer-term (more than 30-day) follow-up with no late adverse events [30]. We think that follow-up studies should be extended up to 1 year or longer to detect late adverse events, e.g., pancreatic duct stricture formation or the development of chronic pancreatitis.
These findings show the short-term safety and efficacy of TPS and also highlight the necessity of long-term follow-up studies after precut papillotomy.
References
Testoni PA, Mariani A, Aabakken L, et al. Papillary cannulation and sphincterotomy techniques at ERCP: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2016;48:657–683.
Liao WC, Angsuwatcharakon P, Isayama H, et al. International consensus recommendations for difficult biliary access. Gastrointest Endosc. 2017;85:295–304.
Gyokeres T, Duhl J, Varsanyi M, Schwab R, Burai M, Pap A. Double guide wire placement for endoscopic pancreaticobiliary procedures. Endoscopy. 2003;35:95–96.
Goff JS. Common bile duct pre-cut sphincterotomy: transpancreatic sphincter approach. Gastrointest Endosc. 1995;41:502–505.
Tse F, Yuan Y, Moayyedi P, Leontiadis GI, Barkun AN. Double-guidewire technique in difficult biliary cannulation for the prevention of post-ERCP pancreatitis: a systematic review and meta-analysis. Endoscopy. 2017;49:15–26.
Pécsi D, Farkas N, Hegyi P, et al. Transpancreatic sphincterotomy has a higher cannulation success rate than needle-knife precut papillotomy—a meta-analysis. Endoscopy. 2017;49:874–887.
Kubota K, Sato T, Kato S, et al. Needle-knife precut papillotomy with a small incision over a pancreatic stent improves the success rate and reduces the complication rate in difficult biliary cannulations. J Hepatobiliary Pancreat Sci. 2013;20:382–388.
Madacsy L, Kurucsai G, Fejes R, Szekely A, Szekely I. Prophylactic pancreas stenting followed by needle-knife fistulotomy in patients with sphincter of Oddi dysfunction and difficult cannulation: new method to prevent post-ERCP pancreatitis. Dig Endosc. 2009;21:8–13.
Horiuchi A, Nakayama Y, Kajiyama M, Tanaka N. Effect of precut sphincterotomy on biliary cannulation based on the characteristics of the major duodenal papilla. Clin Gastroenterol Hepatol. 2007;5:1113–1118.
Goff JS. Long-term experience with the transpancreatic sphincter pre-cut approach to biliary sphincterotomy. Gastrointest Endosc. 1999;50:642–645.
Kozarek R. Flail, flay, or fail: needle-knife versus transpancreatic sphincterotomy to access the difficult-to-cannulate bile duct during ERCP. Endoscopy. 2017;49:842–843.
Kozarek RA, Ball TJ, Patterson DJ, Brandabur JJ, Traverso LW, Raltz S. Endoscopic pancreatic duct sphincterotomy: indications, technique, and analysis of results. Gastrointest Endosc. 1994;40:592–598.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269.
Wells GA SB, O’Connell D, Peterson J, Welch V, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. 2011; Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Higgins JP, Altman DG, Gøtzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188.
Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions, vol. 4. New York: Wiley; 2011.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–634.
Cha S-W, Kim SH, Kim A, et al. 447 DGT versus TPS in patients with initial PD cannulation by chance; prospective randomized multi-center study. Gastrointest Endosc. 2012;75:AB141.
Sugiyama H, Tsuyuguchi T, Sakai Y, et al. Transpancreatic precut papillotomy versus double-guidewire technique in difficult biliary cannulation: prospective randomized study. Endoscopy. 2018;50:33–39.
Yoo YW, Cha SW, Lee WC, Kim SH, Kim A, Cho YD. Double guidewire technique versus transpancreatic precut sphincterotomy in difficult biliary cannulation. World J Gastroenterol. 2013;19:108–114.
Kim CW, Chang JH, Kim TH, Han SW. Sequential double-guidewire technique and transpancreatic precut sphincterotomy for difficult biliary cannulation. Saudi J Gastroenterol. 2015;21:18–24.
Zou XP, Leung JW, Li YH, et al. Comparison of sequential pancreatic duct guidewire placement technique and needle knife precut sphincterotomy for difficult biliary cannulation. J Dig Dis. 2015;16:741–746.
Catalano MF, Linder JD, Geenen JE. Endoscopic transpancreatic for inaccessible obstructed papillary septotomy bile ducts: comparison with standard pre-cut papillotomy. Gastrointest Endosc. 2004;60:557–561.
Zang J, Zhang C, Gao J. Guidewire-assisted transpancreatic sphincterotomy for difficult biliary cannulation: a prospective randomized controlled trial. Surg Laparosc Endosc Percutaneous Tech. 2014;24:429–433.
Espinel-Diez J, Pinedo-Ramos E, Vaquero-Ayala L, Alvarez-Cuenllas B, Ojeda-Marrero V. Combined precut in difficult biliary cannulation. Rev Esp Enferm Dig. 2013;105:334–337.
Katsinelos P, Gkagkalis S, Chatzimavroudis G, et al. Comparison of three types of precut technique to achieve common bile duct cannulation: a retrospective analysis of 274 cases. Dig Dis Sci. 2012;57:3286–3292. https://doi.org/10.1007/s10620-012-2271-8.
Lee YJ, Park YK, Lee MJ, Lee KT, Lee KH, Lee JK. Different strategies for transpancreatic septotomy and needle knife infundibulotomy due to the presence of unintended pancreatic cannulation in difficult biliary cannulation. Gut Liver. 2015;9:534–539.
Wen J, Li T, Gong B. Efficacy and safety of transpancreatic septotomy, needle-knife fistulotomy or both selected based on unintentional pancreatic access and papillary morphology. J Dig Dis. 2017;18:41.
Kahaleh M, Tokar J, Mullick T, Bickston SJ, Yeaton P. Prospective evaluation of pancreatic sphincterotomy as a precut technique for biliary cannulation. Clin Gastroenterol Hepatol. 2004;2:971–977.
Weber A, Roesch T, Pointner S, et al. Transpancreatic precut sphincterotomy for cannulation of inaccessible common bile duct: a safe and successful technique. Pancreas. 2008;36:187–191.
Akashi R, Kiyozumi T, Jinnouchi K, Yoshida M, Adachi Y, Sagara K. Pancreatic sphincter precutting to gain selective access to the common bile duct: a series of 172 patients. Endoscopy. 2004;36:405–410.
Barakat MT, Girotra M, Huang RJ, et al. Goff trans-pancreatic septotomy is an effective and safe salvage technique following failed standard biliary cannulation at ERCP. Gastrointest Endosc. 2017;85:AB606.
Chan CH, Brennan FN, Zimmerman MJ, Ormonde DG, Raftopoulos SC, Yusoff IF. Wire assisted transpancreatic septotomy, needle knife precut or both for difficult biliary access. J Gastroenterol Hepatol. 2012;27:1293–1297.
de-la-Morena-Madrigal EJ. Impact of combined precut techniques on selective biliary cannulation. Rev Esp Enferm Dig. 2013;105:338–344.
de-la-Morena-Madrigal EJ, Rodriguez Garcia MF, Galera Rodenas AB, Perez Arellano E. Biliary cannulation effectiveness and pancreatitis risk using two early precut techniques. Rev Esp Enferm Dig. 2018;110:74–81.
Esmaily S, Elzubier M, Dwarakanath D, et al. Transpancreatic sphincterotomy: a valuable technique for gaining CBD access. United Eur Gastroenterol J. 2017;5:A697.
Halttunen J, Keranen I, Udd M, Kylanpaa L. Pancreatic sphincterotomy versus needle knife precut in difficult biliary cannulation. Surg Endosc. 2009;23:745–749.
Huang C, Kung J, Liu Y, et al. Use of double wire-guided technique and transpancreatic papillary septotomy in difficult ERCP: 4-year experience. Endosc Int Open. 2016;4:E1107–E1110.
Javia SB, Priyanka P, Avila N, et al. Transpancreatic sphincterotomy (Goff septotomy) is safe and effective in patients with failed wire/contrast guided biliary cannulation. Gastrointest Endosc. 2016;83:AB606.
Kapetanos D, Kokozidis G, Christodoulou D, et al. Case series of transpancreatic septotomy as precutting technique for difficult bile duct cannulation. Endoscopy. 2007;39:802–806.
Liao C, Park W, Chen A, Friedland S, Banerjee S. Goff trans-pancreatic septotomy is an effective and safe biliary cannulation technique for patients who fail standard biliary cannulation. Am J Gastroenterol. 2011;106:S56–S56.
Lin LF. Transpancreatic precut sphincterotomy for biliary access: the relation of sphincterotomy size to immediate success rate of biliary cannulation. Diagn Ther Endosc. 2014;2014:864082.
McGonigle J, Mitra V, Dwarakanath D, Chaudhury B, Majumdar D, Hancock J. The safety and efficacy of transpancreatic sphincterotomy for difficult CBD cannulation during ERCP. Pancreatology. 2014;14:S25–S26.
Miao L, Li QP, Zhu MH, et al. Endoscopic transpancreatic septotomy as a precutting technique for difficult bile duct cannulation. World J Gastroenterol. 2015;21:3978–3982.
Miyatani H, Yoshida Y. Endoscopic needle knife precut papillotomy for inaccessible bile duct following failed pancreatic duct access. Clin Med Gastroenterol. 2009;2009:1–5.
Wang P, Zhang W, Liu F, et al. Success and complication rates of two precut techniques, transpancreatic sphincterotomy and needle-knife sphincterotomy for bile duct cannulation. J Gastrointest Surg. 2010;14:697–704.
Zhong H, Wang X, Yang L, Miao L, Ji G, Fan Z. Modified transprepancreatic septotomy reduces postoperative complications after intractable biliary access. Medicine (Baltimore). 2018;97:e9522.
Cotton P, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–393.
Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53):1119–1129.
Rashdan A, Fogel EL, McHenry L Jr, Sherman S, Temkit M, Lehman GA. Improved stent characteristics for prophylaxis of post-ERCP pancreatitis. Clin Gastroenterol Hepatol. 2004;2:322–329.
Lawrence C, Cotton PB, Romagnuolo J, Payne KM, Rawls E, Hawes RH. Small prophylactic pancreatic duct stents: an assessment of spontaneous passage and stent-induced ductal abnormalities. Endoscopy. 2007;39:1082–1085.
Gódi S, Pécsi D, Hegyi P, et al. Initial experiences with transpancreatic sphincterotomy in Hungarian centers based on prospectively collected registry data. Endoscopy. 2019;51:S76.
Acknowledgments
Open access funding provided by University of Pécs (PTE).
Funding
Funding was provided from Economic Development and Innovation Operative Programme Grant of the National Research, Development and Innovation Office (GINOP-2.3.2-15-2016-00048), and from the ÚNKP-17-3-I New National Excellence Program of the Ministry of Human Capacities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pécsi, D., Farkas, N., Hegyi, P. et al. Transpancreatic Sphincterotomy Is Effective and Safe in Expert Hands on the Short Term. Dig Dis Sci 64, 2429–2444 (2019). https://doi.org/10.1007/s10620-019-05640-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05640-4