Abstract
Background
Eradication therapies for Helicobacter pylori infection are advancing as new acid inhibitory reagents approved. The aim of this study was to assess the efficacy and safety of vonoprazan-based triple treatment.
Materials and Methods
Triple therapy with vonoprazan and two antibiotics (amoxicillin and clarithromycin or metronidazole) received focus in this analysis. We performed a multicenter retrospective study of patients who received vonoprazan-based eradication therapy between February 2015 and February 2016 and conducted a review of the literature.
Results
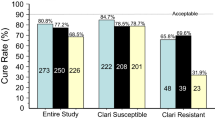
The eradication rate among the 799 patients in our multicenter study was 94.4% (95% confidence interval [CI] 92.6–96.2%) in the per-protocol analysis for first-line treatment (with vonoprazan 20 mg, amoxicillin 750 mg, and clarithromycin 200 or 400 mg, twice a day for 7 days) and 97.1% (95% CI 93.0–101.1%) for second-line treatment (with vonoprazan 20 mg, amoxicillin 750 mg, and metronidazole 250 mg, twice a day for 7 days). The overall incidence of adverse events was 4.4% in an intention-to-treat analysis with no patients hospitalized. In a literature review, six reports, in which 1380 patients received vonoprazan-based first-line eradication therapy, were included and were all reported by Japanese researchers. The eradication success rates in per-protocol analysis were between 85 and 93%, which was roughly the same among the studies.
Conclusions
Vonoprazan-based triple therapy was effective and safe for Helicobacter pylori eradication in real-world experience, confirmed by a multicenter study and a review of the pertinent literature.

Similar content being viewed by others
References
Lee SY. Current progress toward eradicating Helicobacter pylori in East Asian countries: differences in the 2013 revised guidelines between China, Japan, and South Korea. World J Gastroenterol. 2014;20:1493–1502.
Asaka M, Kato M, Takahashi S, et al. Japanese Society for Helicobacter Research. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter. 2010;15:1–20.
Japanese Society for Helicobacter Research. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 revised edition. (Japanese article) Tokyo Japan: Sentan-igakusha; 2016: 46-58.
Inatomi N, Matsukawa J, Sakurai Y, Otake K. Potassium-competitive acid blockers: advanced therapeutic option for acid-related diseases. Pharmacol Ther. 2016;168:12–22.
Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut. 2016;65:1439–1446.
Suzuki S, Gotoda T, Kusano C, Iwatsuka K, Moriyama M. The efficacy and tolerability of a triple therapy containing a potassium-competitive acid blocker compared with a 7-day PPI-based low-dose clarithromycin triple therapy. Am J Gastroenterol. 2016;111:949–956.
Shichijo S, Hirata Y, Niikura R, et al. Vonoprazan versus conventional proton pump inhibitor-based triple therapy as first-line treatment against Helicobacter pylori: a multicenter retrospective study in clinical practice. J Dig Dis. 2016;17:670–675.
Shinozaki S, Nomoto H, Kondo Y, et al. Comparison of vonoprazan and proton pump inhibitors for eradication of Helicobacter pylori. Kaohsiung J Med Sci. 2016;32:255–260.
Noda H, Noguchi S, Yoshimine T, et al. A Novel potassium-competitive acid blocker improves the efficacy of clarithromycin-containing 7-day triple therapy against Helicobacter pylori. J Gastrointestin Liver Dis. 2016;25:283–288.
Matsumoto H, Shiotani A, Katsumata R, et al. Helicobacter pylori eradication with proton pump inhibitors or potassium-competitive acid blockers: the effect of clarithromycin resistance. Dig Dis Sci. 2016;61:3215–3220. doi:10.1007/s10620-016-4305-0.
Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011;11:15.
Otake K, Sakurai Y, Nishida H, et al. Characteristics of the novel potassium-competitive acid blocker vonoprazan fumarate (TAK-438). Adv Ther. 2016;33:1140–1157.
Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212–239.
Sakurai Y, Shiino M, Okamoto H, Nishimura A, Nakamura K, Hasegawa S. Pharmacokinetics and safety of triple therapy with vonoprazan, amoxicillin, and clarithromycin or metronidazole: a phase 1, open-label, randomized, crossover study. Adv Ther. 2016;33:1519–1535.
Fallone CA, Chiba N, van Zanten SV, et al. The toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 2016;151:51–69.
Lee JY, Kin N, Kim MS, et al. Factors affecting first-line therapy of Helicobacter pylori including CYP2C19 genotype and antibiotic resistance. Dig Dis Sci. 2014;59:1235–1243. doi:10.1007/s10620-014-3093-7.
Malfertheiner P, Mégraud F, O’Morain C, et al. The European Helicobacter pylori Study Group (EHPSG). Current European concepts in the management of Helicobacter pylori infection–the Maastricht Consensus Report. Eur J Gastroenterol Hepatol.. 1997;9:1–2.
Malfertheiner P, Megraud F, O’Morain CA, et al.; European Helicobacter Study Group. Management of Helicobacter pylori infection–the maastricht IV/florence consensus report. Gut 2012; 61: 646–664.
Inaba T, Iwamuro M, Toyokawa T, Okada H. Promising results of Helicobacter pylori eradication with vonoprazan-based triple therapy after failure of proton pump inhibitor-based triple therapy. Aliment Pharmacol Ther. 2016;43:179–180.
Katayama Y, Toyoda K, Kusano Y, et al. Efficacy of vonoprazan-based second-line Helicobacter pylori eradication therapy in patients for whom vonoprazan-based first-line treatment failed. Gut 2016; doi:10.1136/gutjnl-2016-312028.
Fukuda D, Akazawa Y, Takeshima F, Nakao K, Fukuda Y. Safety and efficacy of Vonoprazan-based triple therapy against Helicobacter pylori infection: a single-center experience with 1118 patients. Therap Adv Gastroenterol. 2016;9:747–748.
Graham DY. Vonoprazan Helicobacter pylori eradication therapy: ethical and interpretation issues. Gut. 2017;66:384–386.
Yeo YH, Shiu SI, Ho HJ, et al.; Taiwan Gastrointestinal Disease and Helicobacter Consortium. First-line Helicobacter pylori eradication therapies in countries with high and low clarithromycin resistance: a systematic review and network meta-analysis. Gut. 2016; doi:10.1136/gutjnl-2016-311868.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests.
Rights and permissions
About this article
Cite this article
Tanabe, H., Ando, K., Sato, K. et al. Efficacy of Vonoprazan-Based Triple Therapy for Helicobacter pylori Eradication: A Multicenter Study and a Review of the Literature. Dig Dis Sci 62, 3069–3076 (2017). https://doi.org/10.1007/s10620-017-4664-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4664-1