Abstract
Background
Microbiotical dysbiosis induced by a Western diet seems to be associated with an increased risk of developing colorectal cancer (CRC). Few other factors with an effect on the colonic microbiota and their association with CRC have been evaluated.
Aim
We investigated whether the use of antibiotics is associated with CRC risk.
Methods
Data on the use of antibiotics and comedication were extracted from a health insurance database for subjects with a diagnostic-related group for CRC between 2006 and 2011 and four age- and sex-matched controls. Antibiotic use was categorized according to the number of prescriptions during a 5-year follow-up period (1–6 years prior to CRC). Multivariable conditional binary logistic regression analysis was used to estimate odds ratios (ORs) and 95 % confidence intervals (95 % CIs) for different levels of use.
Results
A total of 4029 cases (47 % male, mean age at diagnosis 71 ± 11 years) and 15,988 controls were included. Antibiotics had been prescribed to 2630 (65.3 %) cases and 10,234 (64.0 %) controls (p = 0.13). An increasing use of antibiotics was associated with an increasing risk of CRC [multivariable OR for high (≥8 prescriptions) vs. no prescriptions: 1.26, 95 % CI 1.11–1.44, p-trend <0.01]. For each increase of 5 prescriptions, the OR for CRC was 1.05 (95 % CI 1.01–1.09).
Conclusion
We found an association between the use of antibiotics, especially when used frequently, and the risk of developing CRC. Further studies are needed to establish under which conditions the use of antibiotics increases the risk of developing CRC.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the third most common cancer in men and second most common cancer in women worldwide [1]. Most cases of CRC develop according to the adenoma–carcinoma sequence, which is characterized by the accumulation of genetic and epigenetic mutations leading to benign premalignant lesions and eventually cancer [2]. Important risk factors associated with CRC include lifestyle factors, such as smoking, limited physical activity, and obesity, and a Western diet which is rich in animal fat, red and processed meat, and poor in fibers [3].
In the past few years, an increasing interest has emerged on the role of the gut microbiota in the development of CRC. The human microbiota consists of approximately 1014 bacterial cells of 500–1000 different species and is important for the defense against pathogens, the metabolization of polysaccharides, the production of certain vitamins and plays a key role in maintaining a healthy immune system [4]. Furthermore, the colonic microbiota ferments undigested carbohydrates from fibers into short-chain fatty acids including butyrate, acetate, and propionate [5, 6] which are the preferred energy source of the colon mucosa and possess anti-inflammatory, anti-proliferative, and anti-carcinogenic properties [7–14]. High intakes of meat and animal fat, on the other hand, increase the bacterial production of genotoxic hydrogen sulfide and the secretion of bile acids which are metabolized into carcinogenic secondary bile acids by 7α-dehydroxylating bacteria [15, 16].
Only a few studies have investigated the association between the colonic microbiota and CRC development. These studies show important differences in the composition of the colon microbiota between low and high CRC risk populations based on their diet. For example, a reduced number of short-chain fatty acid-producing bacteria, such as the anti-inflammatory Faecalibacterium prausnitzii from the Clostridium cluster IV and Eubacterium/Roseburia species from cluster XIVa [17], and an increase in secondary bile acid-producing species are found in high CRC risk populations [15, 18]. Furthermore, in CRC patients compared to healthy controls, short-chain fatty acid-producing bacteria have been found to be depleted [19, 20] while the proinflammatory Fusobacterium and Porhyromonas genera were increased [19]. In another study in which stools of CRC patients and healthy subjects were investigated, an increase in Bacteroides–Prevotella populations was demonstrated compared to healthy controls [21].
These findings suggest a potentially important role for the gut microbiota in the development of CRC. It may well be possible that other factors, besides diet, known to induce a disbalance of the gut microbiota are associated with an increased risk of developing CRC. In this regard, the use of antibiotics may be of interest since its use may seriously affect the diversity of the colonic microbiota. We therefore hypothesize that the (frequent) use of antibiotics is associated with an increased CRC risk. In the current study, we investigated whether the use of antibiotics and specific classes of antibiotics is associated with the risk of developing CRC in a population-based cohort. Secondly, we investigated potential effect modification by other factors that have an effect on the gut microbiota or have been found to be associated with CRC risk.
Methods
Data Collection
For this nested case–control study, we used data of the Achmea Health Database in the Netherlands, which is a healthcare claim database covering approximately 1.2 million subjects (8 % of the Dutch population). The database contains anonymized data on demographic characteristics, reimbursed diagnostic-related groups (DRGs), and medication. The population insured by the Achmea Health Insurance Company represents the urbanized area of the Netherlands with regard to age, gender, and socioeconomic status [22].
DRGs were introduced in the Netherlands in 2006 and are based on the International Classification of Disease, 9th revision (ICD-9). They are reimbursed per episode of care provided by secondary care physicians for inpatient and outpatient hospital care services. Data on DRGs were available between January 2006 and December 2011 and contained information on the colorectal cancer diagnosis and date of DRG registration, which usually is the first visit to the physician but can also be a follow-up visit.
Data on reimbursed medication were available between January 2001 and December 2011 and contained information on type of drug (ATC codes), date the drug was filled, number of daily defined doses (DDDs), the prescribed daily dose (PDD), and the prescribing physician, i.e., primary or secondary care. The DDD is the average maintenance dose per day for a drug used for its main indication in adults and is defined by the WHO Collaborating Centre for Drug Statistics Methodology [23]. The PDD is the fraction of DDD per day that is actually prescribed by the treating physician. In the Netherlands, antibiotics can only be obtained with a prescription of a physician and these prescriptions are registered in the Achmea Health Database for subjects insured with this insurance company. However, medication prescriptions during hospitalizations are not registered in the database.
This study was approved by the scientific and privacy committee of the Achmea Health Insurance Company and was performed in accordance with the ethical guidelines of our institute.
Study Population
The complete database was searched for adult (≥18 years) subjects with a DRG for CRC between January 2006 and December 2011. An incidence CRC case was defined as a subject with at least two DRGs for CRC or one DRG for CRC surgery, in which the first DRG was not registered within the first 1.5 years of follow-up. This 1.5-year clean period was chosen to minimize the risk of including prevalent CRC cases and was based on the recommended follow-up of patients with CRC every 6–12 months until 5 years after initial treatment with curative intent or more frequently in case of palliative treatment (according to the national guidelines at that time [24, 25]). The date of CRC diagnosis was defined as the date of first DRG registration. Each case was matched with regard to sex and date of birth to four randomly selected controls without a DRG for CRC and with at least the same period of follow-up as their matched case. Both cases and controls were required to have at least 6 years of complete follow-up before CRC diagnosis. Cases and controls that at some point during follow-up had a DRG for inflammatory bowel disease were excluded.
Antibiotic Use
Antibiotics included were tetracyclines (ATC codes J01A), amphenicols (ATC codes J01B), penicillins (ATC codes J01C), cephalosporins (ATC codes J01D), sulfonamides and trimethoprim (ATC codes J01E), macrolides (ATC codes J01F), aminoglycosides (ATC codes J01G), quinolones (ATC codes J01M), imidazoles (ATC codes J01XD), nitrofuran derivates (ATC codes J01XE), and others (ATC codes J01XA, J01XB, J01XC, J01XX). The number of days for which antibiotics were prescribed was calculated as prescribed days = DDD/PDD. For prescriptions with an unknown DDD (3.6 %) or PDD (7.7 %), values were imputed with SAS PROC MI procedure, under the missing at random assumption and based on ATC code, primary or secondary care prescribing physician, sex, and age. The use of antibiotics was measured as the number of prescriptions and the prescribed number of days during a 5-year period in the period 1–6 years prior to CRC diagnosis. Subjects were categorized as nonusers and very low (1st–50th percentile), low (51st–75th percentile), intermediate (76th–90th percentile), and high (above 90th percentile) users of antibiotics. For the analyses of anti-anaerobic agents and subtypes of antibiotics, we categorized subjects as nonusers and low (1st–75th percentile), intermediate (76th–90th percentile), and high (above 90th percentile) users.
Covariates
Covariates included in this study were sex, age (continuous), insulin-dependent diabetes (ATC codes A10A, no/yes), insulin-independent diabetes (ATC codes A10B, no/yes), and the use of proton pump inhibitors (ATC codes A02BC), acetylsalicylic acids (ATC codes B01AC06 and B01AC08), nonsteroidal anti-inflammatory drugs (ATC codes M01A), lipid-lowering agents (ATC codes C10AA, C10BA, and C10BX), estrogens (ATC codes G03AA, G03AB, G03FA, and G03FB), and immunosuppressive drugs (ATC codes L04A). The cumulative number of prescribed days per drug was categorized as none and the 1st–75th, 76th–90th, and above 90th percentile within users.
Statistical Analyses
The use of antibiotics and comedication in cases and matched controls was assessed over a 5-year period between 1 and 6 years prior to CRC diagnosis. Medication use within 1 year prior to CRC diagnosis was not included in the analysis to minimize the risk of reversed causation. Differences in baseline characteristics were compared between cases and controls and expressed in means ± standard deviations (SDs), medians (interquartile range—IQR), and frequencies whenever applicable. Student’s t test and Mann–Whitney U test were used for continuous variables and Pearson Chi-square test for categorical variables.
Univariable and multivariable binary logistic regression analyses, conditioned on the matching factors sex and date of birth, were used to calculate the odds ratio (OR) and 95 % confidence intervals (95 % CIs) for the use of antibiotics on a categorical and continuous scale and the risk of developing CRC. Linear trends over different categories were computed using median levels of antibiotic use within the categories in all subjects. Two models were tested: (1) a univariable conditioned model on the matching factors age and sex and (2) a multivariable model conditioned on these matching factors and adjusted for factors statistically significantly associated with the outcome or the use of antibiotics.
Effect modification between the use of antibiotics and other factors that may affect the gut microbiota or that have previously been found to be associated with CRC risk was tested by adding multiplicative interaction terms to the model and using likelihood ratio tests for interaction. For these analyses, we used the interaction terms of overall antibiotic use (number of prescriptions, categorical) with insulin-independent diabetes (no/yes), insulin-dependent diabetes (no/yes), proton pump inhibitors (no/yes), acetylsalicylic acids (no/yes), nonsteroidal anti-inflammatory drugs (no/yes), statins (no/yes), estrogens (no/yes), and immunosuppressive drugs (no/yes).
Sensitivity analyses were performed to study possible reversed causation by assessing the use of antibiotics between 2–7 years and 3–8 years prior to CRC diagnosis.
Statistical analyses were conducted with SAS 9.2 (SAS Institute Inc, Cary, USA). Two-sided p values <0.05 were considered statistically significant.
Results
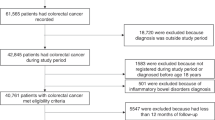
Between January 2006 and December 2011, 8141 subjects were identified with a DRG for CRC. After the exclusion of subjects with a DRG for inflammatory bowel disease (n = 88), prevalent CRC cases (n = 3518), or subjects with less than 6 years of follow-up (n = 506) before the first DRG, 4029 incident CRC cases remained available for the analysis. These cases were matched to 15,988 controls without inflammatory bowel disease or CRC.
Baseline Characteristics
Baseline characteristics are shown in Table 1. Of all CRC cases, 47 % were male and mean age at diagnosis was 71 ± 11 years. No statistically significant differences were found between cases and controls with regard to insulin-independent (p = 0.32) and insulin-dependent (p = 0.45) diabetes and use of proton pump inhibitors (p = 0.14), acetylsalicylic acids (p = 0.09), lipid-lowering agents (p = 0.56), estrogens (p = 0.48), and immunosuppressive drugs (p = 0.52). However, subjects with CRC used less nonsteroidal anti-inflammatory drugs (p < 0.01) compared to controls. All these covariates were statistically significantly (p < 0.001) associated with an increasing use of antibiotics.
The most frequently prescribed antibiotics included penicillins (31.6 %), tetracyclines (20.7 %), quinolones (13.9 %), macrolides (9.6 %), sulfonamides and trimethoprim (9.6 %), and nitrofuran derivates (12.4 %). Other antibiotics, including cephalosporins, aminoglycosides, amphenicols, and imidazoles, were rarely prescribed (combined 2.2 %). During the 5-year follow-up period, antibiotics had been prescribed to 2630 (65.3 %) CRC cases and 10,234 (64.0 %) controls (p = 0.13). When excluding nonusers, the median number of prescriptions of antibiotics was 2 (IQR 1–5) versus 2 (1–5) times (p = 0.07) for cases and controls, respectively, corresponding to 17 (IQR 8–35) versus 16 (8–34) days of use (p = 0.10).
Use of Antibiotics and Colorectal Cancer Risk
A high (≥8) number of prescriptions of antibiotics were associated with an increased risk of CRC (see Table 2). Univariable OR was 1.23 (95 % CI 1.08–1.40, p-trend <0.01) when comparing a high (≥8) number versus no prescriptions and was 1.04 (95 % CI 1.01–1.07) for each increase of 5 prescriptions. Multivariable analyses adjusted for all measured potential confounders showed an OR of 1.26 (95 % CI 1.11–1.44, p-trend <0.01) for a high (≥8) number versus no prescriptions and an OR of 1.05 (95 % CI 1.01–1.09) for each increase of 5 prescriptions.
The analyses for the number of prescribed days yielded a statistically significantly increased risk of CRC when antibiotics were used for ≥70 days versus no use of antibiotics (univariable OR 1.24, 95 % CI 1.08–1.44, p-trend <0.01; multivariable OR 1.28, 95 % CI 1.10–1.48, p-trend <0.01). However, on a continuous scale (per 25 days increase), no association was found (multivariable OR 1.00, 95 % CI 0.99–1.01).
When specified for anti-aerobic and anti-anaerobic antibiotics (see Table 3), we found a positive association between the number of prescriptions and the risk of CRC for both anti-aerobic [multivariable high (≥8) vs. no prescriptions 1.25, 95 % CI 1.08–1.45] and anti-anaerobic agents [multivariable high (≥5) vs. no prescriptions 1.45, 95 % CI 1.07–1.97]. When further categorized by classes of antibiotics, we found an increased risk for penicillins [multivariable high (≥5) vs. no prescriptions 1.29, 95 % CI 1.06–1.56] and quinolones [multivariable high (≥5) vs. no prescriptions 1.53, 95 % CI 1.19–1.96], but not for tetracyclines, sulfonamides and trimethoprim, macrolides, and nitrofuran derivates.
Interaction with Other Factors
No statistically significant interactions were observed between the use of antibiotics and insulin-dependent diabetes (p = 0.14), insulin-independent diabetes (p = 0.84), and the use of proton pump inhibitors (p = 0.59), acetylsalicylic acids (p = 0.62), nonsteroidal anti-inflammatory drugs (p = 0.46), lipid-lowering agents (p = 0.96), estrogens (p = 0.57), or immunosuppressive drugs (p = 0.76).
Sensitivity Analysis
Sensitivity analyses to assess for reversed causation between the use of antibiotics and CRC showed that the OR increased when a less recent follow-up period was used. Multivariable ORs were 1.33 (95 % CI 1.08–1.64) for ≥8 versus 0 prescriptions and 1.17 (95 % CI 0.95–1.43) for ≥70 versus 0 days for antibiotics between 7 and 2 years and 1.37 (95 % CI 1.10–1.70) for ≥8 versus 0 prescriptions and 1.24 (95 % CI 1.00–1.53) for ≥70 versus 0 days for antibiotics between 8 and 3 years prior to CRC diagnosis.
Discussion
The results of this nested case–control study indicate that the use of antibiotics is associated with a dose-dependent increased risk of developing CRC. The results were similar for models adjusted for sex and age and models additionally adjusted for comorbidities and comedication. These positive associations were found for both anti-aerobic and anti-anaerobic drugs; however, when stratified for different classes of antibiotics, we observed only statistically significant associations for penicillins and quinolones.
Previous studies investigating the association between the use of antibiotics and cancer risk demonstrated similar results. A Finnish cohort study including over three million subjects found relative risks of 1.37 (95 % CI 1.34–1.40) for developing any cancer and 1.15 (95 % CI 1.04–1.26) for developing colon cancer when comparing subjects with ≥6 prescriptions to those with 0–1 prescriptions over a 3-year period prior to cancer diagnosis [26]. In a recent nested case–control study in which Boursi et al. included over twenty thousand cases, a dose-dependent increased CRC risk for subjects who had used penicillins, cephalosporins, sulfonamides/trimethoprim, and nitroimidazoles in the 1–5 years prior to index date [27]. Their findings remained significant after adjusting for potential confounding lifestyle factors, comorbidities, comedication, and previous screening colonoscopy. Another nested case–control study by Wang et al. in almost 28,000 patients with type 2 diabetes also found a positive association between the use of anti-anaerobic antibiotics and both colon (OR 2.31, 95 % CI 2.12–2.52) and rectal cancer (OR 1.69, 95 % CI 1.50–1.90), but no association was found for anti-aerobic agents [28]. In contrast, in our study, we found a positive association between the use of anti-aerobic agents and developing CRC, although this was less pronounced when compared to anti-anaerobic agents. The gut microbiota is predominately composed of anaerobes, and the findings reported by Wang et al. and those of our study suggest that particularly the use of anti-anaerobic agents may promote colorectal tumor growth, although caution is required when drawing conclusions about possible explanatory mechanisms.
Whether the observed associations between antibiotics and cancer risk reflect a causal relation is unclear. Antibiotics generally have no known genotoxic potential, and evidence of possible carcinogenic effects of antibiotics is limited [29]. However, some antibiotics up-regulate cyclooxygenase-2 and increase the production of prostaglandins [30], which are important in inflammatory responses and are known to promote the development of CRC [31]. Furthermore, it has been hypothesized that a depletion of anti-inflammatory and short-chain fatty acid-producing species, such as Faecalibacterium prausnitzii and Roseburia, and the abundance of pro-inflammatory microorganisms, such as Fusobacterium, Porhyromonas, Enterococcaceae, and Bacteroides–Prevotella, and toxin-producing species including B. fragilis and some E. coli strains could change the gut microbiota in a more pro-carcinogenic environment [27]. This shift is thought to be dependent on subjects’ age, diet, and pathogen infections, but it can be hypothesized that the frequent use of antibiotics may alter short-chain fatty acid-producing species by causing a dysbiosis of the colonic microbiota, thereby increasing the risk of developing CRC [7–14]. The observed association between the use of antibiotics and CRC risk in our study could also be caused by unmeasured confounding factors associated with CRC risk and a higher use of antibiotics, such as socioeconomic status, smoking, body mass index, and other lifestyle factors. This is supported by the relative modest risk estimates that we found in this study. Nonetheless, when we adjusted for potential confounding factors associated with lifestyle, including diabetes and the use of statins, acetylsalicylic acids, and nonsteroidal anti-inflammatory drugs, the results remained similar. Similarly, modest risk estimates were found in the study by Boursi et al. after adjusting for lifestyle factors, comorbidities, comedication, and previous screening colonoscopy. An alternative explanation for the findings of the present study could be that subjects with a weakened immune system are more susceptible for developing cancer and more frequently develop infections requiring antibiotics. In that case, the observed associations may only be an indicator for an increased cancer risk in general rather than an effector. This may be why in previous studies positive associations have been found between the use of antibiotics and tumors in other organs, such as breast, prostate, lung, and thyroid cancer and non-Hodgkin’s lymphoma, which, as far as we currently understand, do not have an association with the gut microbiota [26, 32–36]. We observed no statistically significant interaction between the use of antibiotics and other factors that may affect the gut microbiota or that have previously been reported to be associated with CRC risk, which suggests that there is no synergetic effect of antibiotics with diabetes or other medications. We also did not find evidence for reversed causation since prolonging the lag time between the last time point of antibiotic use and CRC diagnosis from 1 to 2 or 3 years had no effect on the results. On the contrary, the risk estimates increased when a follow-up period further back in time was employed. This increases the likelihood of a causal effect since CRC has been estimated to develop over a period of 8–10 years [37].
The strengths of this study include the large number of CRC cases and matched controls that were identified in a health claim database providing high-quality data on the use of antibiotics and potential confounding medication and comorbidity. In the Netherlands, antibiotics can only be obtained with a prescription of a physician and therefore are registered in the health claim database. Both DRG and pharmacy registrations are complete and highly accurate because of the economic function of the database for the insurance company [22]. Furthermore, compared to most previous studies, we included a higher number of subjects from the general population [26, 28, 32].
A limitation of our study is that the diagnosis of CRC could not be histologically confirmed. Second, as previously mentioned, there were no data available on potential confounding lifestyle factors, which is a limitation of the present study. Third, antibiotics prescribed during hospitalizations are not registered in the database. Therefore, we could not include the antibiotics that are more frequently prescribed during hospitalization, such as cephalosporins, aminoglycosides, and imidazoles. There were also no data available on whether the dispensed antibiotics were actually used. Both may have diluted the true size of the association between antibiotics and CRC risk. Finally, we did not have data on the microbiota composition of the subjects included in our study. Combined data on the use of antibiotic agents and the composition of the gut microbiota could be of additive value to further detangle the association between antibiotic use and CRC development. Nevertheless, the dose-dependent increase and the similar risk estimates for antibiotic use up to 8 years before diagnosis suggest that a dysbiosis of the gut microbiota through the use of antibiotics may partially contribute to the development of CRC.
As of now, there is insufficient evidence to make a clinical recommendation regarding the use of antibiotics and CRC risk. The results of our study do, however, support the idea that a microbiotical disbalance in the colorectum may increase the risk of developing CRC. Two previous studies, of which one in a general population investigating a shorter exposure period and one only including subjects with type 2 diabetes, found a positive association between the use of antibiotics and CRC risk. These findings are now supported by our study in a general population. Additional epidemiological studies with long-term follow-up should focus on the mutual effects of antibiotic use and lifestyle factors to further elucidate the association between antibiotic use and CRC risk. Furthermore, observational studies including measurements of the (changes in) microbiota composition in relation to CRC risk may provide more insights in the role of the gut microbiota in the pathogenesis of CRC.
In conclusion, we observed a positive association between the use of antibiotics, especially when used frequently, and the risk of developing CRC. Whether this association resembles a causal relation must be investigated in future studies.
References
Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917.
Weitz J, Koch M, Debus J, et al. Colorectal cancer. Lancet. 2005;365:153–165.
World Cancer Research Fund/American Institute for Cancer Research. Systematic Literature Review Continuous Update Project Report. 2011 (Ref Type: Internet Communication).
Sommer F, Backhed F. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–238.
Augenlicht LH, Mariadason JM, Wilson A, et al. Short chain fatty acids and colon cancer. J Nutr. 2002;132:3804S–3808S.
Scheppach W. Effects of short chain fatty acids on gut morphology and function. Gut. 1994;35:S35–S38.
Cox MA, Jackson J, Stanton M, et al. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J Gastroenterol. 2009;15:5549–5557.
Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133:2485S–2493S.
Ebert MN, Klinder A, Peters WH, et al. Expression of glutathione S-transferases (GSTs) in human colon cells and inducibility of GSTM2 by butyrate. Carcinogenesis. 2003;24:1637–1644.
Greer JB, O’Keefe SJ. Microbial induction of immunity, inflammation, and cancer. Front Physiol. 2011;1:168.
Kim YS, Milner JA. Dietary modulation of colon cancer risk. J Nutr. 2007;137:2576S–2579S.
Nguyen KA, Cao Y, Chen JR, et al. Dietary fiber enhances a tumor suppressor signaling pathway in the gut. Ann Surg. 2006;243:619–625.
Rodriguez-Cabezas ME, Galvez J, Lorente MD, et al. Dietary fiber down-regulates colonic tumor necrosis factor alpha and nitric oxide production in trinitrobenzenesulfonic acid-induced colitic rats. J Nutr. 2002;132:3263–3271.
Waldecker M, Kautenburger T, Daumann H, et al. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J Nutr Biochem. 2008;19:587–593.
Ou J, Carbonero F, Zoetendal EG, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013;98:111–120.
Vipperla K, O’Keefe SJ. The microbiota and its metabolites in colonic mucosal health and cancer risk. Nutr Clin Pract. 2012;27:624–635.
Wells JE, Hylemon PB. Identification and characterization of a bile acid 7alpha-dehydroxylation operon in Clostridium sp. strain TO-931, a highly active 7alpha-dehydroxylating strain isolated from human feces. Appl Environ Microbiol. 2000;66:1107–1113.
Ou J, Delany JP, Zhang M, et al. Association between low colonic short-chain fatty acids and high bile acids in high colon cancer risk populations. Nutr Cancer. 2012;64:34–40.
Ahn J, Sinha R, Pei Z, et al. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013;105:1907–1911.
Balamurugan R, Rajendiran E, George S, et al. Real-time polymerase chain reaction quantification of specific butyrate-producing bacteria, Desulfovibrio and Enterococcus faecalis in the feces of patients with colorectal cancer. J Gastroenterol Hepatol. 2008;23:1298–1303.
Sobhani I, Tap J, Roudot-Thoraval F, et al. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS One. 2011;6:e16393.
Smeets HM, de Wit NJ, Hoes AW. Routine health insurance data for scientific research: potential and limitations of the Agis Health Database. J Clin Epidemiol. 2011;64:424–430.
WHO Collaborating Centre for Drug Statistics Methodology. 2012. http://www.whocc.no.
Dutch National Guideline for Colon Cancer. CBO; 2008.
Dutch National Guideline for Rectal Cancer. CBO; 2008.
Kilkkinen A, Rissanen H, Klaukka T, et al. Antibiotic use predicts an increased risk of cancer. Int J Cancer. 2008;123:2152–2155.
Candela M, Turroni S, Biagi E, et al. Inflammation and colorectal cancer, when microbiota-host mutualism breaks. World J Gastroenterol. 2014;20:908–922.
Wang JL, Chang CH, Lin JW, et al. Infection, antibiotic therapy and risk of colorectal cancer: a nationwide nested case-control study in patients with Type 2 diabetes mellitus. Int J Cancer. 2014;135:956–967.
Snyder RD. An update on the genotoxicity and carcinogenicity of marketed pharmaceuticals with reference to in silico predictivity. Environ Mol Mutagen. 2009;50:435–450.
Attur MG, Patel RN, Patel PD, et al. Tetracycline up-regulates COX-2 expression and prostaglandin E2 production independent of its effect on nitric oxide. J Immunol. 1999;162:3160–3167.
Dixon DA, Blanco FF, Bruno A, et al. Mechanistic aspects of COX-2 expression in colorectal neoplasia. Recent Results Cancer Res. 2013;191:7–37.
Didham RC, Reith DM, McConnell DW, et al. Antibiotic exposure and breast cancer in New Zealand. Breast Cancer Res Treat. 2005;92:163–167.
Kato I, Koenig KL, Baptiste MS, et al. History of antibiotic use and risk of non-Hodgkin’s lymphoma (NHL). Int J Cancer. 2003;107:99–105.
Tamim HM, Hajeer AH, Boivin JF, et al. Association between antibiotic use and risk of prostate cancer. Int J Cancer. 2010;127:952–960.
Velicer CM, Heckbert SR, Lampe JW, et al. Antibiotic use in relation to the risk of breast cancer. JAMA. 2004;291:827–835.
Zhang H, Garcia Rodriguez LA, Hernandez-Diaz S. Antibiotic use and the risk of lung cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:1308–1315.
Risio M. The natural history of adenomas. Best Pract Res Clin Gastroenterol. 2010;24:271–280.
Acknowledgments
The authors thank Henk Evers (Achmea Health Insurance, Amersfoort, the Netherlands) for helping with collecting the data.
Author contributions
Study concept and design: Dik, Smeets, Siersema. Acquisition of data: all authors. Data analyses: Dik. Drafting of the manuscript: Dik. Critical revision of the manuscript for important intellectual content: all authors. Study supervision: Van Oijen, Siersema.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dik, V.K., van Oijen, M.G.H., Smeets, H.M. et al. Frequent Use of Antibiotics Is Associated with Colorectal Cancer Risk: Results of a Nested Case–Control Study. Dig Dis Sci 61, 255–264 (2016). https://doi.org/10.1007/s10620-015-3828-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3828-0