Abstract
Background
Ion channels and transporters are potential markers and therapeutic targets for several cancers. However, their expression during hepatocellular carcinoma (HCC) development remains unclear.
Aim
To investigate the mRNA expression of Na+, K+ and Ca2+ channels and ABC transporters during rat HCC development, as well as Abcc3 protein in human liver biopsies.
Methods
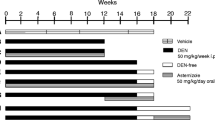
Wistar rats were treated with diethylnitrosamine (DEN) and developed both cirrhosis (12 weeks of treatment) and either pre-neoplastic lesions (16 weeks of treatment) or multinodular HCC (16 weeks of treatment plus 2 weeks DEN-free). The mRNA expression of 12 ion channels and two ABC transporters was studied using real-time RT-PCR. Tumor-containing or tumor-free liver sections were isolated by laser-capture microdissection. Abcc3 protein expression was studied by immunohistochemistry in healthy, cirrhotic and HCC human biopsies.
Results
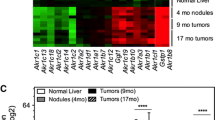
We observed expression changes in seven genes. Kcna3, Kcnn4, Kcnrg and Kcnj11 potassium channel mRNA expression reached peak values at the end of DEN treatment, while Scn2a1 sodium channel, Trpc6 calcium channel and Abcc3 transporter mRNA expression reached their highest levels in the presence of HCC (18 weeks). Whereas Kcnn4 and Scn2a1 channel expression was similar in non-tumor and tumor tissue, the Abcc3 transporter and Kcna3 potassium channels were preferentially overexpressed in the tumor sections. We observed differential Abcc3 protein subcellular localization and expression in human samples.
Conclusions
The ion channel/transporter expression profile observed suggests that these genes are potential early markers or therapeutic targets of HCC. The differential localization of Abcc3 may be useful in the diagnosis of cirrhosis and HCC.





Similar content being viewed by others
References
Globocan. International Agency for Research on Cancer, 2012. https://globocan.iarc.fr. Accessed 22 June 2013.
Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346.
Heindryckx F, Colle I, Van Vlierberghe H. Experimental mouse models for hepatocellular carcinoma research. Int J Exp Pathol. 2009;90:367–386.
Farber E. The multistep nature of cancer development. Cancer Res. 1984;44:4217–4223.
Liu YF, Zha BS, Zhang HL, et al. Characteristic gene expression profiles in the progression from liver cirrhosis to carcinoma induced by diethylnitrosamine in a rat model. J Exp Clin Cancer Res. 2009;28:107.
Schiffer E, Housset C, Cacheux W, et al. Gefitinib, an EGFR inhibitor, prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology. 2005;41:307–314.
Paradis V. Histopathology of hepatocellular carcinoma. Rec Results Cancer Res. 2013;190:21–32.
Filmus J, Capurro M. Glypican-3: a marker and a therapeutic target in hepatocellular carcinoma. FEBS J. 2013;280:2471–2476.
Lee JI, Lee JW, Kim JM, et al. Prognosis of hepatocellular carcinoma expressing cytokeratin 19: comparison with other liver cancers. World J Gastroenterol. 2012;18:4751–4757.
Pardo LA, del Camino D, Sanchez A, et al. Oncogenic potential of EAG K(+) channels. EMBO J. 1999;18:5540–5547.
Cuddapah VA, Sontheimer H. Ion channels and transporters in cancer. 2. Ion channels and the control of cancer cell migration. Am J Physiol Cell Physiol. 2011;301:C541–C549.
Camacho J. Ether a go-go potassium channels and cancer. Cancer Lett. 2006;233:1–9.
Rodriguez-Rasgado JA, Acuna-Macias I, Camacho J. Eag1 channels as potential cancer biomarkers. Sensors (Basel). 2012;12:5986–5995.
Hemmerlein B, Weseloh RM, Mello de Queiroz F, et al. Overexpression of Eag1 potassium channels in clinical tumours. Mol Cancer. 2006;5:41.
Pardo LA, Stuhmer W. The roles of K(+) channels in cancer. Nat Rev Cancer. 2014;14:39–48.
Diaz L, Ceja-Ochoa I, Restrepo-Angulo I, et al. Estrogens and human papilloma virus oncogenes regulate human ether-a-go-go-1 potassium channel expression. Cancer Res. 2009;69:3300–3307.
Ousingsawat J, Spitzner M, Puntheeranurak S, et al. Expression of voltage-gated potassium channels in human and mouse colonic carcinoma. Clin Cancer Res. 2007;13:824–831.
Zhang L, Barritt GJ. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 2004;64:8365–8373.
Ortiz CS, Montante-Montes D, Saqui-Salces M, et al. Eag1 potassium channels as markers of cervical dysplasia. Oncol Rep. 2011;26:1377–1383.
Makino H, Uetake H, Danenberg K, et al. Efficacy of laser capture microdissection plus RT-PCR technique in analyzing gene expression levels in human gastric cancer and colon cancer. BMC Cancer. 2008;8:210.
Curran S, McKay JA, McLeod HL, et al. Laser capture microscopy. Mol Pathol. 2000;53:64–68.
Torres Mena JE, Sanchez Rodriguez R, Quintanar Jurado V, et al. Laser capture microdissection after gamma-glutamyl transferase histochemistry: an optimization for gene expression analysis. Anal Biochem. 2014;447:126–132.
Rutenburg AM, Kim H, Fischbein JW, et al. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969;17:517–526.
Pompella A, De Tata V, Paolicchi A, et al. Expression of gamma-glutamyltransferase in cancer cells and its significance in drug resistance. Biochem Pharmacol. 2006;71:231–238.
Djamgoz MB, Coombes RC, Schwab A. Ion transport and cancer: from initiation to metastasis. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130092.
Arcangeli A, Crociani O, Bencini L. Interaction of tumour cells with their microenvironment: ion channels and cell adhesion molecules. A focus on pancreatic cancer. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130101.
Nies AT, Konig J, Pfannschmidt M, et al. Expression of the multidrug resistance proteins MRP2 and MRP3 in human hepatocellular carcinoma. Int J Cancer. 2001;94:492–499.
Urrego D, Tomczak AP, Zahed F, et al. Potassium channels in cell cycle and cell proliferation. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130094.
Oliveira PA, Colaco A, Chaves R, et al. Chemical carcinogenesis. An Acad Bras Cienc. 2007;79:593–616.
Ohya S, Niwa S, Kojima Y, et al. Intermediate-conductance Ca2+-activated K+ channel, KCa3.1, as a novel therapeutic target for benign prostatic hyperplasia. J Pharmacol Exp Ther. 2011;338:528–536.
Freise C, Ruehl M, Seehofer D, et al. The inhibitor of Ca(2+)-dependent K+ channels TRAM-34 blocks growth of hepatocellular carcinoma cells via downregulation of estrogen receptor alpha mRNA and nuclear factor-kappaB. Invest New Drugs. 2013;31:452–457.
Jang SH, Kang KS, Ryu PD, et al. Kv1.3 voltage-gated K(+) channel subunit as a potential diagnostic marker and therapeutic target for breast cancer. BMB Rep. 2009;42:535–539.
Wu J, Zhong D, Wu X, et al. Voltage-gated potassium channel Kv1.3 is highly expressed in human osteosarcoma and promotes osteosarcoma growth. Int J Mol Sci. 2013;14:19245–19256.
Ivanov DV, Tyazhelova TV, Lemonnier L, et al. A new human gene KCNRG encoding potassium channel regulating protein is a cancer suppressor gene candidate located in 13q14.3. FEBS Lett. 2003;539:156–160.
Cho YG, Kim CJ, Song JH, et al. Genetic and expression analysis of the KCNRG gene in hepatocellular carcinomas. Exp Mol Med. 2006;38:247–255.
Cai R, Ding X, Zhou K, et al. Blockade of TRPC6 channels induced G2/M phase arrest and suppressed growth in human gastric cancer cells. Int J Cancer. 2009;125:2281–2287.
Fraser SP, Ozerlat-Gunduz I, Brackenbury WJ, et al. Regulation of voltage-gated sodium channel expression in cancer: hormones, growth factors and auto-regulation. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130105.
Brisson L, Gillet L, Calaghan S, et al. Na(V)1.5 enhances breast cancer cell invasiveness by increasing NHE1-dependent H(+) efflux in caveolae. Oncogene. 2011;30:2070–2076.
Gillet L, Roger S, Besson P, et al. Voltage-gated sodium channel activity promotes cysteine cathepsin-dependent invasiveness and colony growth of human cancer cells. J Biol Chem. 2009;284:8680–8691.
Szakacs G, Paterson JK, Ludwig JA, et al. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234.
Scheffer GL, Kool M, de Haas M, et al. Tissue distribution and induction of human multidrug resistant protein 3. Lab Invest. 2002;82:193–201.
Young LC, Campling BG, Cole SP, et al. Multidrug resistance proteins MRP3, MRP1, and MRP2 in lung cancer: correlation of protein levels with drug response and messenger RNA levels. Clin Cancer Res. 2001;7:1798–1804.
Wang XZ, Chen ZX, Zhang LJ, et al. Expression of insulin-like growth factor 1 and insulin-like growth factor 1 receptor and its intervention by interleukin-10 in experimental hepatic fibrosis. World J Gastroenterol. 2003;9:1287–1291.
Anilkumar TV, Golding M, Edwards RJ, et al. The resistant hepatocyte model of carcinogenesis in the rat: the apparent independent development of oval cell proliferation and early nodules. Carcinogenesis. 1995;16:845–853.
Sell S. Heterogeneity and plasticity of hepatocyte lineage cells. Hepatology. 2001;33:738–750.
Acknowledgments
We thank Eduardo García Osornio, M.V.Z. Ricardo Gaxiola and Rafael Leyva for their technical assistance. This work was partially supported by the Consejo Nacional de Ciencia y Tecnología grant 168102 to JC and grant 115431 to JIP-C.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This is a retrospective study, and for this type of study, formal consent is not required.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zúñiga-García, V., Chávez-López, M.d.G., Quintanar-Jurado, V. et al. Differential Expression of Ion Channels and Transporters During Hepatocellular Carcinoma Development. Dig Dis Sci 60, 2373–2383 (2015). https://doi.org/10.1007/s10620-015-3633-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3633-9