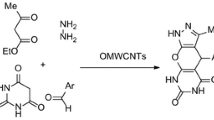
Abstract
We report here the formation of carbon–carbon bonds via carbon-hydrogen bond activation catalysed by multi-walled carbon nanotubes (mwcnts), the catalytic activity of which is influenced by nanocarbon morphology and structure. Control of nanocarbon defects and edges allows the realisation of a high-performance carbon-based catalyst that can replace its metal-based counterparts.
Graphic Abstract







Similar content being viewed by others
Notes
The reaction produces isomeric o-, m-, and p-substituted products in a 4:1:5 ratio regardless of the conditions.
MWCNTs (1 g) were placed in a ceramic container and heated in an electric furnace at 850 °C for 4 h in air. The residue (~ 1 mg) was analyzed by EDX, leading to the detection of Fe and other metals (Fig. S1).
Although XPS did not show the difference before and after mCPBA treatment, thermo-gravimetric analysis (TGA) showed increased weight loss for ox-MWCNTs at 200 °C and 530 °C. Such an increased weight loss would suggest the formation of oxygenated functional groups and defects on MWCNTs. Results of TGA and zeta potential analysis of the recovered MWCNT are also shown in Fig S2 and Table S1.
Products of homolytic C–C, C–O, and O–O bond cleavage of mCPBA (m-chlorobenzoic acid, benzyl m-chlorobenzoate, 3,3'-dichloro-1,1'-biphenyl) were observed in the reaction mixture. Therefore, the type of radical mainly contributing to the reaction is unclear.
References
Su DS, Wen G, Wu S, Peng F, Schlögl R (2016) Angew Chem Int Ed 55:2
Yu JQ, Shi Z (2010) Top Curr Chem 292:35–56
Liu C, Yuan J, Gao M, Tang S, Li W, Shi R, Lei A (2015) Chem Rev 115:12138
Perea-Buceta JEPB, Wirtanen T, Laukkanen OV, Mäkelä MK, Nieger M, Melchionna M, Huittinen N, Lopez-Sanchez JA, Helaja J (2013) Angew Chem Int Ed 52(45):11835–11839
Sun CL, Shi ZJ (2014) Chem Rev 114: 9219
Liu X, Dai L (2016) Nat Rev Mater 1:16064
Wirtanen T, Makela MK, Sarfraz J, Ihalainen P, Hietala S, Melchionna M, Helaja J (2015) Adv Synth Catal 357:3718–3726
Melchionna M, Marchesan S, Prato M, Fornasiero P (2015) Catal Sci Technol 5:3859–3875
Gao Y, Tang P, Zhou H, Zhang W, Yang H, Yan N, Hu G, Mei D, Wang J, Ma D (2016) Angew Chem Int Ed 55:3124
Morioku K, Morimoto N, Takeuchi Y, Nishina Y (2016) Sci Rep 6:25824
Prileschajew N (1909) Ber 42:4811
Hoveyda AH, Evans DA, Fu GC (1983) Chem Rev 93:1307
Larsen AS, Wang K, Lockwood MA, Rice GL, Won TJ, Lovell S, Sadílek M, Tureček F, Mayer JM (2002) J Am Chem Soc 124:10112
Evnin AB, Lam AY (1968) Chem Commun 1968:1184
Kondo T, Tantayanon S, Tsuji Y, Watanabe Y (1989) Tetrahedron Lett 31:4137
Uemura S, Tanaka S, Okano M (1976) J Chem Soc Perkin Trans 1:1966
King ST (1991) J Catal 131:215
Pumera M (2007) Langmuir 23:6453–6458
Chen ML, Oh WC (2011) Nanoscale Res Lett 6:398
Shen A, Zou Y, Wang Q, Dryfe RAW, Huang X, Dou S, Dai L, Wang S (2014) Angew Chem Int Ed 53:10804
Bartlett PD (1950) Rec Prog Chem 11:47
Singleton DA, Merrigan SR, Liu J, Houk KN (1997) J Am Chem Soc 119:3385
Bravo A, Fontana F, Minisci F, Serri A (1996) Chem Commun 1996:1843
Sheldon RA, Arends IWCE, Brink GJT, Dijksman A (2002) Acc Chem Res 35:774
Cuesta A, Dhamelincourt P, Laureyns J, Alonso AM, Tascón JMD (1994) Carbon 32:1523
Cristarella TC, Chinderle AJ, Hui J, Rodriguez-Lopez J (2015) Langmuir 31:3999
Acknowledgements
The authors gratefully acknowledge W. Chen for his contribution to XPS measurements, T. Yamazaki for his contribution to ESR measurements and H. Suzuki for his contribution to NMR. This work was financially supported by JST SICORP, ANR (ANR-15-JTIC-0002-01), and the Egyptian government.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
El-Hout, S.I., Zhou, Y., Kano, J. et al. Dehydrogenative Coupling of Toluene Promoted by Multi-Walled Carbon Nanotubes. Catal Lett 150, 256–262 (2020). https://doi.org/10.1007/s10562-019-02951-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02951-z