Abstract
We assessed the left atrial-left ventricular (LA-LV) long axis angulation value as a new measure of LA remodeling, and studied its predictors, its effect on two-dimensional LA volume (2D LAVol) estimation, and optimization techniques for 2D LAVol values. Retrospective electrocardiogram-gated coronary computed tomographic angiograms of 164 consecutive patients were reviewed. The LA–LV angle was measured in reconstructed 3-chamber views, and its predictors were determined. The LAVol measured by the area-length method after image optimization along the LV long axis (AL) and the LA long axis (AC–AL), was compared with that measured by the three-dimensional (3D)-volumetric method. LAVol calculation was modified to minimize differences from the 3D values. LA–LV angles ranged from 0° to 63°. In the univariate analysis, decreasing angulation was significantly associated with increasing LV end-diastolic volume (LVEDV), mitral regurgitation grade, LV and LA anteroposterior dimensions, and decreasing LV ejection fraction (LVEF). On multivariate analysis, increasing LVEDV, MR, and LA anteroposterior dimension inversely correlated with angulation; LVEF was positively correlated. The AL and 3D methods significantly differed only for patients with angles ≤ 29.9°. Conversely, LAVol was overestimated for all angules by AC–AL. Modification of AL LAVol using a regression equation, or by substituting the shortest with the longest and average LA lengths in patients with angles ≤ 29.9° and 30–39.9°, respectively neutralized the difference. The LA–LV angle is a new measure of LA and LV remodeling predicted by LV size and function, MR, and LA-anteroposterior dimension. AL formula modifications based on angulation in LV-optimized views better correlate with the 3D method than LA-view modification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A recent interest in the malalignment of the left atrial (LA) long axis relative to that of the left ventricle (LV) has surfaced, particularly in relation to its potential implications on LA volume (LAVol) determination by two dimensional-transthoracic echocardiography (2D-TTE) [1,2,3] which has been used to obtain most normative and predictive LAVol data [1, 4, 5]. In the clinical practice, the 4-chamber and 2-chamber 2D-TTE views are routinely optimized along the LV long axis such that an unrecognized angulation during scanning may lead to LA foreshortening and underestimation of LAVol by 2D-TTE [1, 3, 5, 6]. While obtaining extra views along the LA long axis has been advocated [1, 3], the value of this approach and its techniques are yet to be clearly established [1, 7].
Additionally, current knowledge of this parameter is limited and its predictors have not been investigated. It has been speculated that elevation of the LV apex by the diaphragm in older individuals may contribute to increased angulation [8] but no evidence exists on this potential mechanism. Further investigation of this parameter is required as it may add to and improve the present measures of LA remodeling [9, 10] namely 2D LAVol. LA structural remodeling is complex, involves more than enlargement of the LA [11], and is related to LV remodeling [12]. Improved understanding of LA remodeling and its mechanisms provides valuable diagnostic, prognostic, and therapeutic insights into the management of patients with cardiac conditions [13].
Computed tomographic angiography (CTA) enables accurate and validated determination of LAVol [14] and can also determine LA–LV angulation and perform image optimization along the LA long axis with the use of proprietary software. This study aimed to assess the clinical and anatomical predictors of LA–LV angulation, and its effect on LAVol measurement by the standard area-length (AL) method, and techniques for the optimization of AL LAVol calculation based on angulation degree.
Methods
Patient population
This study enrolled 164 consecutive patients who had undergone both a clinically indicated retrospective electrocardiogram (ECG)-gated coronary CTA and 2D-TTE within a 15-day period from January 2008 to October 2010 at the Ottawa Heart Institute. These patients were enrolled in a registry and their clinical and imaging data including age, sex, height, weight, LV end-diastolic volume (LVEDV), and LV ejection fraction (LVEF) by CTA, were prospectively recorded. Patients with atrial fibrillation, congenital heart disease, mechanical mitral prosthesis, or history of cardiac transplantation were excluded from the analysis. The baseline characteristics of the participants have been described in a previous study [15]. CTA data of 13 originally registered patients were missing and therefore were excluded from this analysis. The study was approved by the Institutional Human Research Ethics Board.
Coronary computed tomography angiography data
Coronary CTAs were performed using a GE Volume CT (GE Healthcare, Milwaukee, WI). Retrospective ECG-gated datasets were obtained using 64 × 0.625 mm slice collimation. A single-segment reconstruction algorithm was performed and ten phases (5–95%) with 1.25 mm slice thickness with 0.625 mm increments were reconstructed for the LA measurements.
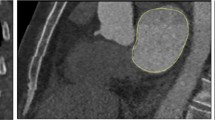
Using CTA, the angulation degree of the LA long axis relative to that of the LV in a reconstructed 3-chamber view was measured using the proprietary software. The LA–LV angle was the intercept angle between the LA long-axis and the LV long-axis, using a view that optimized the LV and LA lengths and demonstrated the LV inflow and outflow tracts akin to the echocardiographic parasternal long-axis view (Fig. 1c).
Measurement of LA–LV angulation and LAVol using the AL method before and after correction for LA–LV angulation. Measurement of LAVol using the AL method from images obtained along the long axis of the LV (a and b). c Measurement of LA–LV angle in a reconstructed 3-chamber view using the proprietary software. d Image reconstruction for optimization of the LA long axis (solid red line). Acquisition of the new LA dimensions from 2-chamber (e) and 4-chamber (f) views formatted along the LA long axis according to the LA–LV angle for calculation of the LAVol by the AC–AL. AC-AL Angle corrected area-length, AL Area length, LA Left atrium, LA-LV left atrial-left ventricular, LAVol Left atrial volume, LV Left ventricle
For each patient, a single reader, blinded to the clinical data, measured LA length, and area in the reconstructed 2- and 4-chamber views using images formatted in the following manner: (1) along the long axis of the LV and (2) along the LA long axis after determination of the LA–LV angle (Fig. 1a–f) with the proprietary software. Additionally, LAVol measured by the CT-three-dimensional (3D) volumetric method was used as the reference standard. A semi-automated software with an attenuation–based endocardial border detection, allowing for manual correction, was used for this purpose with exclusion of the LA appendage and pulmonary veins from the 3D-LAVol measurement as described previously [15]. After acquiring all the data, 2D LAVol was calculated for each participant from the AL formula [5]. Data in the LV optimized views were used to calculate the AL LAVol, while data obtained from the LA optimized views were used to calculate the angle corrected-AL (AC–AL) LAVol.
2D-transthoracic echocardiography data
The 2D-TTE of the patients were reviewed. The following were measured once by a single reader blinded to the CTA angle results and in accordance with the current recommendations [5]: the aortic root, the ascending aorta, the LA anteroposterior dimension (LAd), and LV end diastolic dimension (LVEDd) from the parasternal long axis view and right atrial minor axis diameter (RAd) from the apical 4-chamber view. The presence and severity of mitral regurgitation was recorded.
Statistical analysis
Continuous variables are presented as means with standard deviations and median with interquartile range, and categorical variables are presented as frequencies with percentages. Statistical significance was defined as P < 0.05. Demographic variables and LAVols were stratified by LA–LV angle and compared across strata using the Cochran–Armitage or Jonckheere–Terpstra tests as appropriate. The association between LA–LV angles and clinical and anatomical predictors was analyzed using linear regression. Predictors which were significant in the univariate analysis were further included in multivariate linear regression analysis. Difference between the LAVol using 2D and 3D measurements was assessed using a t-test. The Pearson correlation coefficient was also used to assess the association between the 2D and 3D measures. Analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Result
Values and predictors of LA-LV angles
A total of 164 patients were included in the study. Baseline characteristics of the study population stratified according to the LA–LV angles are summarized in Table 1. Mean age was 58.5 (± 13.8) years and 62.8% were men. The LA–LV angles ranged from 0° to 63° (mean 31.9 ± 12.3°) in this population. A significant decreasing trend was observed for LA–LV angulation and increasing CTA LVEDV (P = 0.039), and LA anteroposterior diameter in the 2D-TTE parasternal long axis view (P < 0.001) (Table 1). The trend between decreasing LA–LV angulation and 3D LAVol, age, sex, CTA LVEF, body surface area (BSA), body mass index (BMI), and mitral regurgitation grade was nonsignificant. Similarly, the association between the LA–LV angle and other anatomic measures, namely aortic root, ascending aorta, and right atrial and left ventricular end-diastolic dimensions was nonsignificant.
Tables 2 and 3 show the association between LA–LV angles and the studied variables using univariate and multivariate analyses, respectively. A significant inverse correlation was observed from the univariate analysis between LVEDV, mitral regurgitation grade and anteroposterior LAd and LVEDd, with LA–LV angles. Conversely, a positive correlation between LVEF and LA–LV angulation was detected (Table 2). On multivariate analysis, only increasing LVEDV, LVEF, mitral regurgitation grade and LAd independently correlated with LA–LV angulation (Table 3). Each of the statistically significant LV parameters (LVEF, LVEDV and LVEDd) was evaluated in a separate multivariate analysis model. Although these parameters measure different aspect of the LV, they were highly colinear; therefore only one was evaluated at a time.
Effect of LA-LV angulation on LAVol measurement by the AL method
Table 4 shows the difference in LAVol between the studied 2D measures and 3D volumetric method, categorized by the degrees of angulation. Results of the attempts on minimizing this difference by adjusting the formula are also shown. A significant difference between the standard AL[5] and 3D was detected (2.6 ml [SD: 1.5, 3.8], P < 0.001) in the overall population, but in the angulation subgroups, the difference was only significant in individuals with LA–LV angles between 0° and 29.9° (P < 0.001). In the hybrid AL, recalculating LAVol by substituting the longest [16] and average [17] for the shortest LA length in the formula for those with angles ≤ 29.9° and 30–39.9° respectively resolved the difference (P = 0.698, Table 4). Similarly, modification of the AL LAVol values according to the individual’s angulation degree by applying an equation obtained by linear regression (Reg) using 3D LAVol as the dependent variable (AL (Reg) = 5.0341 + 0.1255 LA–LV angle + 0.7848 AL LAVol) neutralized the difference in all participants (P = 0.063).
Conversely, LAVol was significantly overestimated by AC-AL compared with 3D at all degrees of LA–LV angulation (P < 0.001). Reassessment by dividing by the longest LA length (AC-AL, Max LA length) in all participants decreased the difference, although it was significant (P < 0.001) (Table 4). Overall, correlation (r ≥ 0.89) of all the studied 2D LAVol measures with the reference standard (Fig. 2) was strong.
Scatterplot and correlation between LAVol by the studied 2D and 3D methods using the Pearson correlation. The dashed line is the diagonal line representing the equivalence of the 2D measurements and the 3D. All LAVols are indexed to the body surface area. AC–AL Angle corrected area-length, AL Area-length, 3D 3-dimensional, LA Left atrium, Max. Maximum, Min. Minimum, r Pearson correlation, Reg Regression
Discussion
This study shows that the angle at which the LA intersects the LV varies among individuals, and in this patient population, it ranged from 0° to 63° (mean 31.9 ± 12.3°). Among the studied determinants of LA–LV angulation, significant trends for increasing LVEDV, and LAd were observed with decreasing angulation. Additionally, based on the univariate analysis, a significant positive correlation with LVEF and a significant inverse correlations with LVEDd, LAd, and mitral regurgitation grade were observed. On multivariate analysis, LVEF, LVEDV, LAd and mitral regurgitation grade were independently associated with LA–LV angulation. No significant association was noted for the participants’ age, sex, or diameters of the aortic root, ascending aorta, and right atrium.
We found that only in subjects with small LA–LV angles (0–29.9°), a significant difference existed between standard AL and 3D LAVols with no significant difference in those with angulations ≥ 30.0°. Conversely, LAVol determined by the AC–AL method (image optimization along the LA long axis) was significantly greater than that by the 3D method at all degrees of angulation. Modification of the AL formula, by substituting the shortest by the longest [16] and average [17] LA lengths for those with angulations ≤ 29.9°, and 30–39.9°, respectively (hybrid AL) and modification of the AL LAVols with a regression equation obliterated the difference in all participants. Overall, a strong correlation for all the studied 2D LAVol measurement methods was noted with the reference standard. To the best of our knowledge, the predictors of LA-LV angulation and its effect on LAVol measurement have not been studied previously, and this is the first study to measure and report on this parameter.
LA–LV angulation and its determinants
Decreased LA-LV angulation may be a new additional measure of LA remodeling [9, 18, 19], beyond LAVol, in patients with mitral regurgitation and LV enlargement and dysfunction, and relate LA to LV remodeling [12]. Unlike LAVol, LA–LV angulation was not associated with the participants’ age, sex, or BSA, although both measures related to LVEDV [20], contrary to LA sphericity, LA–LV angulation was not associated with 3D LA volume or the patients sex [9].
A reduced LVEF, increased LVEDV, anteroposterior LA dimension and mitral regurgitation grade were independent predictors of LA–LV angulation in this study. This constellation of cardiac pathology is commonly observed in patients with heart failure and reduced ejection fraction who have enlarged dysfunctional ventricles [21], greater mitral regurgitation [22] and eccentric LA remodeling [23]. A greater association between LA–LV angulation and LAd compared with 3D LAVol was observed. LA enlargement is not symmetrical [24] and the effect of increasing LA size on decreasing angulation appears primarily driven by increases in LAd. The changes in LA–LV angulation may additionally relate to changes in the mitral annular plane. A recent study involving patients with atrial dilatation and atrial functional mitral regurgitation identified horizontal inclination of the mitral annular plane that decreased with surgical plication, along with decrease of the LA dimension and improvement of the regurgitation [25]. The significant association between LA–LV angulation and worsening mitral regurgitation supports this possibility.
The mechanisms behind the changes in angulation in particular are not apparent. There is paucity of data on the geometric interaction of the cardiac chambers in disease states. Anatomically, the LA forms most of the heart’s base and joins the base of the LV at the mitral valve orifice, anteroinferiorly and to the left [26]. The left ventricle in turn slopes from its base in the plane of the atrioventricular groove to the cardiac apex [27]. It may be postulated that some degree of angulation normally exists in the longitudinal axis of the two chambers and with increasing LV volume and displacement, as well as LA remodeling the angulation decreases.
Although increases in LA–LV angulation have been speculated to occur with age [8], we did not observe such an association in this study. All these mechanistic hypotheses including the potential role of thoracic constraints remain to be explored [9].
Effect of LA-LV angulation on LAVol measurement
The standard AL method overestimated mean indexed LAVol by 2.6 ml compared with 3D but the difference was significant only in patients with angles ≤ 20–29.9°. Discrepancies between 2D and 3D LAVols assessed by the same imaging modality have been reported by other investigators, CTA data are, however, limited [15]. Echocardiographic comparisons have shown both significantly larger [3] and smaller [28] 3D LAVols compared to those obtained by 2D. These differences may be attributed to the type of software used, endocardial tracing errors, 2D misalignment of orthogonal apical 4-chamber and apical 2-chamber views [2] and 2D method used, with a tendency of the AL formula to yield larger volumes [29]. Conversely, the AC-AL method significantly overestimated the mean indexed LAVol by 13 ml compared with 3D and at all angles; with the largest difference (16.1 ml) observed in those with angles 40–49.9°. Comparisons of 2D- and 3D-TTE LAVols, obtained with image optimization along LA long axis, have shown smaller 2D LAVol (with the method of discs), compared with 3D. With 2D-TTE however, and despite all attempts, LA foreshortening and underestimation of LA size may be unavoidable due to the constrains related to acoustic access and the lack of a reliable way to verify and exclude LA foreshortening [3]. CTA overcomes most of these limitations which renders it better suited for 2D LAVol validation.
Among the 2D methods, the standard AL method appears to correlate best with 3D [15]. Despite its excellent performance, however, the AL formula has its inherent limitations and may not be universally applicable. In our study, the formula served best at higher angulation degrees but significantly overestimated LAVol in those with LA–LV angles below 30°. Correcting the results of the AL formula by a regression equation or applying the longest, shortest, or average obtained LA length in the AL formula based on angulation degree, resolved this difference, while maintaining the strong correlation with 3D. Both the longest [16] and average [17] atrial lengths have been used previously.The finding that the AL formula performs differently at different angles suggests that the methods used for 2D LAVol assessment may need to be individualized. The Simpson’s and AL methods are highly correlated [17]. Therefore, the validity of the Simpson’s method in atrial optimized views cannot be speculated and verification is warranted as many clinical [30] and prognostic [31,32,33] decisions rely on the LAVol.
The normal values for LAVol have been increased in the recent guidelines based on new, larger volume, and prognostic data [5]. However, it is not clear if the increased cutoff values are due to larger data, or among other, shifting to atrial focused views. LAVols in studies utilizing standard LV [34, 35] and atrial [36] optimized views, appear comparable, and different values were obtained with non-foreshortened atrial views [28, 37]. The lack of use of a standardized imaging technique in dedicated atrial imaging may have contributed to this discrepancy, particularly as atrial optimization was achieved by maximizing LA area [36], length [28, 37], length and base [3] and obtaining atrial focused views in LV optimized images [38, 39].
A small study (n = 30), assessed LAVol by 2D-TTE from both atrial non-foreshortened and standard apical views with comparison with 3D-TTE. Larger LAVols were obtained from the atrial views, and had better correlation with those from 3D-TTE [40]. Similarly, larger LAVols using the angle corrected view and with a high correlation with those from the 3D method were noted in our study, although these values were significantly different from those of the gold standard. In our study, the comparison of LAVol values in quartiles of angulation provided additional clarification. Our comparison, in addition to the larger sample size, has the advantage of the higher spatial resolution of CTA and better control on image formatting as the 2D-TTE machines are not yet equipped with the software we have used. Given the lack of such technology, if LAVol on 2D-TTE is discrepant from the patient’s clinical background, visual estimation of the LA–LV angle in the parasternal long axis, or apical three-chamber view may explain the findings. Modification of the formula instead of the views in 2D-TTE, is expected to avoid the technical complexity, afford greater consistency and reproducibility, as the atrial views have been associated with an interobserver bias [3], and permit retrospective determination of LAVol from standardized 2D-TTE views [34].
In conclusion, LA–LV angulation appears to be a new promising measure of LA and LV geometry that may be useful in the assessment of suspected cardiac disease. LAVol, among others, is affected by the anatomy of the individual, formula used for its calculation, and the operator’s scanning angle, individually or in combination. The effect of LA–LV angulation on the standard AL derived LAVol though statistically significant may not warrant obtaining extra images along the LA long axis. Such approach may be time consuming, and associated with measurement bias and overestimation of LAVol. Instead, adjusting the AL formula or LAVol values based on angulation degree yields more accurate results compared to those from 3D. Further studies in larger populations are required to validate our observations.
Study limitations
The measurements for LAVol were performed by a single reader. A bias may have occurred due to overestimation of the AC–AL components, and consequently, AC LAVols. This situation however reflects an everyday practice, where different scanners and readers perform the measurements and obtaining views off the standard LV axis maybe difficult and may not be reproducible, particularly for the follow up of patients. Additionally, we measured LA–LV angulation in a single plane, and only have information of the anteroposterior plane whereas the LA has a 3D orientation. Studies assessing the associations and effects of LA–LV angulation on LAVol in larger populations are required to validate our observations.
Data availability
Data are available upon reasonable request.
References
Thomas L, Muraru D, Popescu BA, Sitges M, Rosca M, Pedrizzetti G, Henein MY, Donal E, Badano LP (2020) Evaluation of left atrial size and function: relevance for clinical practice. J Am Soc Echocardiogr 33:934–952. https://doi.org/10.1016/j.echo.2020.03.021
Addetia K, Lang RM (2016) Complexities of left atrial analysis: more than meets the eye? Circ Cardiovasc Imaging. https://doi.org/10.1161/CIRCIMAGING.116.005196
Badano LP, Miglioranza MH, Mihaila S, Peluso D, Xhaxho J, Marra MP, Cucchini U, Soriani N, Iliceto S, Muraru D (2016) Left atrial volumes and function by three-dimensional echocardiography: reference values, accuracy, reproducibility, and comparison with two-dimensional echocardiographic measurements. Circ Cardiovasc Imaging. https://doi.org/10.1161/CIRCIMAGING.115.004229
Boyd AC, Thomas L (2014) Left atrial volumes: two-dimensional, three-dimensional, cardiac magnetic resonance and computed tomography measurements. Curr Opin Cardiol 29:408–416. https://doi.org/10.1097/HCO.0000000000000087
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28(1–39):e14. https://doi.org/10.1016/j.echo.2014.10.003
Miyasaka Y, Tsujimoto S, Maeba H, Yuasa F, Takehana K, Dote K, Iwasaka T (2011) Left atrial volume by real-time three-dimensional echocardiography: validation by 64-slice multidetector computed tomography. J Am Soc Echocardiogr 24:680–686. https://doi.org/10.1016/j.echo.2011.03.009
Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS (2006) Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol 47:2357–2363. https://doi.org/10.1016/j.jacc.2006.02.048
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ, Chamber Quantification Writing G, American Society of Echocardiography’s G, Standards C, European Association of E (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiograph’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18:1440–1463. Doi: https://doi.org/10.1016/j.echo.2005.10.005
Mulder MJ, Kemme MJB, Visser CL, Hopman LHGA, van Diemen PA, van de Ven PM, Götte MJW, Danad I, Knaapen P, van Rossum AC, Allaart CP (2020) Left atrial sphericity as a marker of atrial remodeling: Comparison of atrial fibrillation patients and controls. Int J Cardiol 304:69–74. https://doi.org/10.1016/j.ijcard.2020.01.042.
Hoit BD (2014) Left atrial size and function: role in prognosis. J Am Coll Cardiol 63:493–505. https://doi.org/10.1016/j.jacc.2013.10.055
Hoit BD (2017) Left atrial remodeling: more than just left atrial enlargement. Circ Cardiovasc Imaging. https://doi.org/10.1161/CIRCIMAGING.117.006036
Oliver W, Matthews G, Ayers CR, Garg S, Gupta S, Neeland IJ, Drazner MH, Berry JD, Matulevicius S, de Lemos JA (2017) Factors associated with left atrial remodeling in the general population. Circ Cardiovasc Imaging. https://doi.org/10.1161/CIRCIMAGING.116.005047
Qiu D, Peng L, Ghista DN, Wong KKL (2021) Left atrial remodeling mechanisms associated with atrial fibrillation. Cardiovasc Eng Technol. https://doi.org/10.1007/s13239-021-00527-w
Vandenberg BF, Weiss RM, Kinzey J, Acker M, Stark CA, Stanford W, Burns TL, Marcus ML, Kerber RE (1995) Comparison of left atrial volume by two-dimensional echocardiography and cine-computed tomography. Am J Cardiol 75:754–757
Al-Mohaissen MA, Kazmi MH, Chan KL, Chow BJ (2013) Validation of two-dimensional methods for left atrial volume measurement: a comparison of echocardiography with cardiac computed tomography. Echocardiography 30:1135–1142. https://doi.org/10.1111/echo.12253
Madueme P, Hor KN, Germann J, Mazur W, Jefferies JL, Taylor M (2012) Comparison of area-length method by echocardiography versus full volume quantification by cardiac magnetic resonance imaging for the assessment of left atrial volume. J Cardiovasc Magn Reson 14:P297–P297. https://doi.org/10.1186/1532-429X-14-S1-P297
Takagi Y, Ehara S, Okuyama T, Shirai N, Yamashita H, Sugioka K, Kitamura H, Ujino K, Hozumi T, Yoshiyama M (2009) Comparison of determinations of left atrial volume by the biplane area-length and Simpson’s methods using 64-slice computed tomography. J Cardiol 53:257–264. https://doi.org/10.1016/j.jjcc.2008.11.012
Whitaker J, Karady J, Karim R, Tobon-Gomez C, Fastl T, Razeghi O, O’Neill L, Decroocq M, Williams S, Corrado C, Mukherjee RK, Sim I, O’Hare D, Kotadia I, Kolossvary M, Merkely B, Littvay L, Tarnoki AD, Tarnoki DL, Voros S, Razavi R, O’Neill M, Rajani R, Maurovich Horvat P, Niederer S (2021) Standardised computed tomographic assessment of left atrial morphology and tissue thickness in humans. Int J Cardiol Heart Vasc 32:100694. https://doi.org/10.1016/j.ijcha.2020.100694
Varadarajan V, Ambale-Venkatesh B, Hong SY, Habibi M, Ashikaga H, Wu CO, Chen LY, Heckbert SR, Bluemke DA, Lima JAC (2021) Association of longitudinal changes in NT-proBNP with changes in left atrial volume and function: MESA. Am J Hypertens. https://doi.org/10.1093/ajh/hpab018
D’Andrea A, Riegler L, Rucco MA, Cocchia R, Scarafile R, Salerno G, Martone F, Vriz O, Caso P, Calabro R, Bossone E, Russo MG (2013) Left atrial volume index in healthy subjects: clinical and echocardiographic correlates. Echocardiography 30:1001–1007. https://doi.org/10.1111/echo.12217
Sekaran NK, Crowley AL, de Souza FR, Resende ES, Rao SV (2017) The role for cardiovascular remodeling in cardiovascular outcomes. Curr Atheroscler Rep 19:23. https://doi.org/10.1007/s11883-017-0656-z
Praz F, Grasso C, Taramasso M, Baumbach A, Piazza N, Tamburino C, Windecker S, Maisano F, Prendergast B (2019) Mitral regurgitation in heart failure: time for a rethink. Eur Heart J 40:2189–2193. https://doi.org/10.1093/eurheartj/ehz222
Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA (2015) Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail 8:295–303. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001667
Hof I, Arbab-Zadeh A, Scherr D, Chilukuri K, Dalal D, Abraham T, Lima J, Calkins H (2009) Correlation of left atrial diameter by echocardiography and left atrial volume by computed tomography. J Cardiovasc Electrophysiol 20:159–163. https://doi.org/10.1111/j.1540-8167.2008.01310.x
Matsumori M, Kawashima M, Aihara T, Fujisue J, Fujimoto M, Fukase K, Nomura Y, Tanaka H, Murakami H, Mukohara N (2021) Efficacy of left atrial plication for atrial functional mitral regurgitation. Gen Thorac Cardiovasc Surg 69:458–465. https://doi.org/10.1007/s11748-020-01483-3
Whiteman S, Saker E, Courant V, Salandy S, Gielecki J, Zurada A, Loukas M (2019) An anatomical review of the left atrium. Trans Res Anat 17:100052. https://doi.org/10.1016/j.tria.2019.100052.
Whiteman S, Alimi Y, Carrasco M, Gielecki J, Zurada A, Loukas M (2021) Anatomy of the cardiac chambers: a review of the left ventricle. Trans Res Anat 23:100095. https://doi.org/10.1016/j.tria.2020.100095.
Iwataki M, Takeuchi M, Otani K, Kuwaki H, Haruki N, Yoshitani H, Tamura M, Abe H, Otsuji Y (2012) Measurement of left atrial volume from transthoracic three-dimensional echocardiographic datasets using the biplane Simpson’s technique. J Am Soc Echocardiogr 25:1319–1326. https://doi.org/10.1016/j.echo.2012.08.017
Jiamsripong P, Honda T, Reuss CS, Hurst RT, Chaliki HP, Grill DE, Schneck SL, Tyler R, Khandheria BK, Lester SJ (2008) Three methods for evaluation of left atrial volume. Eur J Echocardiogr 9:351–355. https://doi.org/10.1016/j.euje.2007.05.004
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29:277–314. https://doi.org/10.1016/j.echo.2016.01.011
Cho IJ, Jeong H, Choi JY, Lee SE, Chang HJ (2019) Prognostic implications of the left atrial volume index in patients with progressive mitral stenosis. J Cardiovasc Imaging 27:122–133. https://doi.org/10.4250/jcvi.2019.27.e20
Poulsen MK, Dahl JS, Henriksen JE, Hey TM, Hoilund-Carlsen PF, Beck-Nielsen H, Moller JE (2013) Left atrial volume index: relation to long-term clinical outcome in type 2 diabetes. J Am Coll Cardiol 62:2416–2421. https://doi.org/10.1016/j.jacc.2013.08.1622
Moller JE, Hillis GS, Oh JK, Seward JB, Reeder GS, Wright RS, Park SW, Bailey KR, Pellikka PA (2003) Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation 107:2207–2212. https://doi.org/10.1161/01.CIR.0000066318.21784.43
Barnes ME, Miyasaka Y, Seward JB, Gersh BJ, Rosales AG, Bailey KR, Petty GW, Wiebers DO, Tsang TS (2004) Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin Proc 79:1008–1014. https://doi.org/10.4065/79.8.1008
Takemoto Y, Barnes ME, Seward JB, Lester SJ, Appleton CA, Gersh BJ, Bailey KR, Tsang TS (2005) Usefulness of left atrial volume in predicting first congestive heart failure in patients > or = 65 years of age with well-preserved left ventricular systolic function. Am J Cardiol 96:832–836. https://doi.org/10.1016/j.amjcard.2005.05.031
Nistri S, Galderisi M, Ballo P, Olivotto I, D’Andrea A, Pagliani L, Santoro A, Papesso B, Innelli P, Cecchi F, Mondillo S, Working Group on Echocardiography of the Italian Society of C (2011) Determinants of echocardiographic left atrial volume: implications for normalcy. Eur J Echocardiogr 12:826–833. Doi: https://doi.org/10.1093/ejechocard/jer137.
Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, Cha SS, Seward JB (2006) Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol 47:1018–1023. https://doi.org/10.1016/j.jacc.2005.08.077
Kou S, Caballero L, Dulgheru R, Voilliot D, De Sousa C, Kacharava G, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Gomez De Diego JJ, Hagendorff A, Henri C, Hristova K, Lopez T, Magne J, De La Morena G, Popescu BA, Penicka M, Ozyigit T, Rodrigo Carbonero JD, Salustri A, Van De Veire N, Von Bardeleben RS, Vinereanu D, Voigt JU, Zamorano JL, Donal E, Lang RM, Badano LP, Lancellotti P (2014) Echocardiographic reference ranges for normal cardiac chamber size: results from the NORRE study. Eur Heart J Cardiovasc Imaging 15:680–690. https://doi.org/10.1093/ehjci/jet284
Lancellotti P, Badano LP, Lang RM, Akhaladze N, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Gomez de Diego JJ, Derumeaux G, Dulgheru R, Edvardsen T, Galderisi M, Goncalves A, Habib G, Hagendorff A, Hristova K, Kou S, Lopez T, Magne J, de la Morena G, Popescu BA, Penicka M, Rasit T, Rodrigo Carbonero JD, Salustri A, Van de Veire N, von Bardeleben RS, Vinereanu D, Voigt JU, Voilliot D, Zamorano JL, Donal E, Maurer G (2013) Normal reference ranges for echocardiography: rationale, study design, and methodology (NORRE Study). Eur Heart J Cardiovasc Imaging 14:303–308. https://doi.org/10.1093/ehjci/jet008
Kebed K, Kruse E, Addetia K, Ciszek B, Thykattil M, Guile B, Lang RM, Mor-Avi V (2017) Atrial-focused views improve the accuracy of two-dimensional echocardiographic measurements of the left and right atrial volumes: a contribution to the increase in normal values in the guidelines update. Int J Cardiovasc Imaging 33:209–218. https://doi.org/10.1007/s10554-016-0988-8
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Benjamin Chow holds the Saul and Edna Goldfarb Chair in Cardiac Imaging Research. He receives research support from TD Bank, AusculSciences, Siemens Healthineers, and Artrya. He has equity interest in General Electric. The remaining authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Institutional Human Research Ethics Board and all procedures were in accordance with the declaration of Helsinki.
Consent for publication
All authors have given their consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Mohaissen, M.A., Chow, B.J.W., Lee, T. et al. Left atrial-left ventricular angle, a new measure of left atrial and left ventricular remodeling. Int J Cardiovasc Imaging 38, 435–445 (2022). https://doi.org/10.1007/s10554-021-02411-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-021-02411-z