Abstract
Purpose
To investigate the utility of mammography for breast cancer screening in a population of males at increased risk for breast cancer.
Methods
In this HIPAA-compliant institutional review board-approved single-institution study, mammography records and clinical data of 827 male patients who underwent digital mammography from September 2011–July 2018 were analyzed via the electronic medical record. 664 of these men presented with masses, pain, or nipple discharge and were excluded from this study. The remaining 163 asymptomatic men with familial and/or personal history of breast cancer, or with a known germline mutation in BRCA, underwent screening mammography and were included in this analysis.
Results
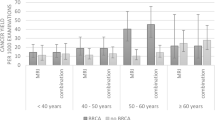
163 asymptomatic men (age: mean 63 years, range 24–87 years) underwent 806 screening mammograms. 125/163 (77%) had a personal history of breast cancer and 72/163 (44%) had a family history of breast cancer. 24/163 (15%) were known mutation carriers: 4/24 (17%) BRCA1 and 20/24 (83%) BRCA2. 792/806 (98%) of the screening mammograms were negative (BI-RADS 1 or 2); 10/806 (1.2%) were classified as BI-RADS 3, all of which were eventually downgraded to BI-RADS 2 on follow-up. 4/806 (0.4%) mammograms were abnormal (BI-RADS 4/5): all were malignant. The cancer detection rate in this cohort was 4.9 cancers/1000 examinations.
Conclusions
In our cohort, screening mammography yielded a cancer detection rate of 4.9 cancers/1000 examinations which is like the detection rate of screening mammography in a population of women at average risk, indicating that screening mammography is of value in male patients at high risk for breast cancer.



Similar content being viewed by others
References
Liu N, Johnson KJ, Ma CX (2018) Male breast cancer: an updated surveillance, epidemiology, and end results data analysis. Clin Breast Cancer 18(5):e997–e1002
Korde LA, Zujewski JA, Kamin L et al (2010) Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol Off J Am Soc Clin Oncol 28:2114–2122. https://doi.org/10.1200/JCO.2009.25.5729
Liede A, Karlan BY, Narod SA (2004) Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol Off J Am Soc Clin Oncol 22:735–742. https://doi.org/10.1200/JCO.2004.05.055
Meijers-Heijboer H, van den Ouweland A, Klijn J et al (2002) Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet 31:55–59. https://doi.org/10.1038/ng879
Evans DB, Crichlow RW (1987) Carcinoma of the male breast and Klinefelter’s syndrome: is there an association? CA Cancer J Clin 37:246–251
Yu X-F, Feng W-L, Miao L-L et al (2013) The prognostic significance of molecular subtype for male breast cancer: a 10-year retrospective study. Breast Edinb Scotl 22:824–827. https://doi.org/10.1016/j.breast.2013.02.005
Piscuoglio S, Ng CKY, Murray MP et al (2016) The genomic landscape of male breast cancers. Clin Cancer Res Off J Am Assoc Cancer Res 22:4045–4056. https://doi.org/10.1158/1078-0432.CCR-15-2840
Vermeulen MA, Slaets L, Cardoso F et al (2017) Pathological characterisation of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Eur J Cancer Oxf Engl 82:219–227. https://doi.org/10.1016/j.ejca.2017.01.034
Cardoso F, Bartlett JMS, Slaets L et al (2018) Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncol Off J Eur Soc Med Oncol 29:405–417. https://doi.org/10.1093/annonc/mdx651
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7–30. https://doi.org/10.3322/caac.21442
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) (2018) Genetic/familial high-risk assessment—breast and ovarian, version 1. https://www.tri-kobe.org/nccn/guideline/gynecological/english/genetic_familial.pdf. (n.d.)
D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA et al (2013) ACR BI-RADS® atlas, breast imaging reporting and data system. American College of Radiology, Reston, VA. http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/BIRADS/BIRADS%20V5%20Changes.pdf. Accessed 20 Sep 2015
Eccles DM, Evans DG, Mackay J (2000) Guidelines for a genetic risk based approach to advising women with a family history of breast cancer. UK Cancer Family Study Group (UKCFSG). J Med Genet 37:203–209
Sardanelli F, Boetes C, Borisch B et al (2010) Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer 46:1296–1316. https://doi.org/10.1016/j.ejca.2010.02.015
Vasen HF, Haites NE, Evans DG et al (1998) Current policies for surveillance and management in women at risk of breast and ovarian cancer: a survey among 16 European family cancer clinics. European Familial Breast Cancer Collaborative Group. Eur J Cancer Oxf Engl 34:1922–1926
Evans DGR, Lalloo F (2002) Risk assessment and management of high risk familial breast cancer. J Med Genet 39:865–871
Eisinger F, Alby N, Bremond A et al (1998) Recommendations for medical management of hereditary breast and ovarian cancer: the French National Ad Hoc Committee. Ann Oncol Off J Eur Soc Med Oncol 9:939–950
Warner E, Heisey RE, Goel V et al (1999) Hereditary breast cancer. Risk assessment of patients with a family history of breast cancer. Can Fam Physician Med Fam Can 45:104–112
Singer CF, Tea M-K, Pristauz G et al (2012) Guideline for the prevention and early detection of breast and ovarian cancer in high risk patients, particularly in women from HBOC (hereditary breast and ovarian cancer) families. Wien Klin Wochenschr 124:334–339. https://doi.org/10.1007/s00508-012-0173-6
Møller P, Evans G, Haites N et al (1999) Guidelines for follow-up of women at high risk for inherited breast cancer: consensus statement from the Biomed 2 Demonstration Programme on Inherited Breast Cancer. Dis Mark 15:207–211
Kuhl CK, Schrading S, Leutner CC et al (2005) Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 23:8469–8476. https://doi.org/10.1200/JCO.2004.00.4960
Leach MO, Boggis CRM, Dixon AK et al (2005) Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet Lond Engl 365:1769–1778. https://doi.org/10.1016/S0140-6736(05)66481-1
Kuhl C, Weigel S, Schrading S et al (2010) Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol Off J Am Soc Clin Oncol 28:1450–1457. https://doi.org/10.1200/JCO.2009.23.0839
Brenner RJ, Weitzel JN, Hansen N, Boasberg P (2004) Screening-detected breast cancer in a man with BRCA2 mutation: case report. Radiology 230:553–555. https://doi.org/10.1148/radiol.2302030360
Dershaw DD (1986) Male mammography. AJR Am J Roentgenol 146:127–131. https://doi.org/10.2214/ajr.146.1.127
Dershaw DD, Borgen PI, Deutch BM, Liberman L (1993) Mammographic findings in men with breast cancer. AJR Am J Roentgenol 160:267–270. https://doi.org/10.2214/ajr.160.2.8424331
Freedman BC, Keto J, Rosenbaum Smith SM (2012) Screening mammography in men with BRCA mutations: is there a role? Breast J 18:73–75. https://doi.org/10.1111/j.1524-4741.2011.01185.x
Mathew J, Perkins GH, Stephens T et al (2008) Primary breast cancer in men: clinical, imaging, and pathologic findings in 57 patients. Am J Roentgenol 191:1631–1639. https://doi.org/10.2214/AJR.08.1076
Anderson WF, Jatoi I, Tse J, Rosenberg PS (2010) Male breast cancer: a population-based comparison with female breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 28:232–239. https://doi.org/10.1200/JCO.2009.23.8162
Weigelt B, Peterse JL, van’t Veer LJ (2005) Breast cancer metastasis: markers and models. Nat Rev Cancer 5:591–602. https://doi.org/10.1038/nrc1670
Fidler IJ (2003) The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat Rev Cancer 3:453–458. https://doi.org/10.1038/nrc1098
Hellman S (1994) Karnofsky memorial lecture. Natural history of small breast cancers. J Clin Oncol Off J Am Soc Clin Oncol 12:2229–2234. https://doi.org/10.1200/JCO.1994.12.10.2229
Michaelson JS, Silverstein M, Wyatt J et al (2002) Predicting the survival of patients with breast carcinoma using tumor size. Cancer 95:713–723. https://doi.org/10.1002/cncr.10742
Gibbs P, Onishi N, Sadinski M et al (2019) Characterization of sub-1 cm breast lesions using radiomics analysis. J Magn Reson Imaging 1:1. https://doi.org/10.1002/jmri.26732
Sopik V, Narod SA (2018) The relationship between tumour size, nodal status and distant metastases: on the origins of breast cancer. Breast Cancer Res Treat 170:647–656. https://doi.org/10.1007/s10549-018-4796-9
Mango V, Bryce Y, Morris EA et al (2018) Commentary ACOG practice bulletin July 2017: breast cancer risk assessment and screening in average-risk women. Br J Radiol 91:20170907. https://doi.org/10.1259/bjr.20170907
Expert Panel on Breast Imaging, Niell BL, Lourenco AP et al (2018) ACR appropriateness criteria® evaluation of the symptomatic male breast. J Am Coll Radiol 15:S313–S320. https://doi.org/10.1016/j.jacr.2018.09.017
Funding
This research was funded in part through the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748 and a Grant from the Breast Cancer Research Foundation. The sponsors were not involved in the study design; the collection, analysis, and interpretation of data; the writing of the report; and the decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KP received payment for activities not related to the present article including lectures including service on speakers bureaus and for travel/accommodations/meeting expenses unrelated to activities listed from the European Society of Breast Imaging (MRI educational course, annual scientific meeting). MSJ has received an honorarium from GE for speaking, and an honorarium for speaking at the Lynn Sage Breast Cancer Symposium and at MD Anderson. EAM has received a grant from GRAIL. The rest of the authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
As this was a retrospective study, the need for informed consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marino, M.A., Gucalp, A., Leithner, D. et al. Mammographic screening in male patients at high risk for breast cancer: is it worth it?. Breast Cancer Res Treat 177, 705–711 (2019). https://doi.org/10.1007/s10549-019-05338-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05338-1