Abstract
Purpose
Tamoxifen is one of the principal treatments for estrogen receptor (ER)-positive breast cancer. Unfortunately, between 30 and 50% of patients receiving this hormonal therapy relapse. Since CYP2D6 genetic variants have been reported to play an important role in survival outcomes after treatment with tamoxifen, this study sought to summarize and critically appraise the available scientific evidence on this topic.
Methods
A systematic literature review was conducted to identify studies investigating associations between CYP2D6 genetic variation and survival outcomes after tamoxifen treatment. Critical appraisal of the retrieved scientific evidence was performed, and recommendations were developed for CYP2D6 genetic testing in the context of tamoxifen therapy.
Results
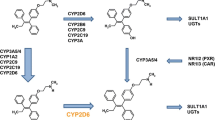
Although conflicting literature exists, the majority of the current evidence points toward CYP2D6 genetic variation affecting survival outcomes after tamoxifen treatment. Of note, review of the CYP2D6 genotyping assays used in each of the studies revealed the importance of comprehensive genotyping strategies to accurately predict CYP2D6 metabolizer phenotypes.
Conclusions and recommendations
Critical appraisal of the literature provided evidence for the value of comprehensive CYP2D6 genotyping panels in guiding treatment decisions for non-metastatic ER-positive breast cancer patients. Based on this information, it is recommended that alternatives to standard tamoxifen treatments may be considered in CYP2D6 poor or intermediate metabolizers.



Similar content being viewed by others
References
Global Burden of Disease Pediatrics Collaboration, Fitzmaurice C, Dicker D et al (2015) The Global Burden of Cancer 2013. JAMA Oncol 1:505–527. https://doi.org/10.1001/jamaoncol.2015.0735
Dunnwald LK, Rossing MA, Li CI (2007) Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res 9:R6. https://doi.org/10.1186/bcr1639
Jordan VC (2003) Tamoxifen: a most unlikely pioneering medicine. Nat Rev Drug Discov 2:205–213. https://doi.org/10.1038/nrd1031
Maximov PY, Lee TM, Jordan VC (2013) The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr Clin Pharmacol 8(2):135–155
Jordan VC (2007) Chemoprevention of breast cancer with selective oestrogen-receptor modulators. Nat Rev Cancer 7(1):46–53
Cole MP, Jones CT, Todd ID (1971) A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Cancer 25:270–275
Early Breast Cancer Trialists’ Collaborative Group, Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level metaanalysis of randomised trials. Lancet 378:771–784. https://doi.org/10.1016/S0140-6736(11)60993-8
Early Breast Cancer Trialists’ Collaborative Group (1998) Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 351:1451–1467
Kiyotani K, Mushiroda T, Nakamura Y, Zembutsu H (2012) Pharmacogenomics of tamoxifen: roles of drug metabolizing enzymes and transporters. Drug Metab Pharmacokinet 27:122–131
Coezy E, Borgna JL, Rochefort H (1982) Tamoxifen and metabolites in MCF7 cells: correlation between binding to estrogen receptor and inhibition of cell growth. Cancer Res 42:317–323
Katzenellenbogen BS, Norman MJ, Eckert RL, Peltz SW, Mangel WF (1984) Bioactivities, estrogen receptor interactions, and plasminogen activator-inducing activities of tamoxifen and hydroxy-tamoxifen isomers in MCF-7 human breast cancer cells. Cancer Res 44:112–119
Barginear MF, Jaremko M, Peter I, Yu C, Kasai Y, Kemeny M, Raptis G, Desnick RJ (2011) Increasing tamoxifen dose in breast cancer patients based on CYP2D6 genotypes and endoxifen levels: effect on active metabolite isomers and the antiestrogenic activity score. Clin Pharmacol Ther 90:605–611. https://doi.org/10.1038/clpt.2011.153
Jordan VC (1982) Metabolites of tamoxifen in animals and man: identification, pharmacology, and significance. Breast Cancer Res Treat 2:123–138
Lim YC, Desta Z, Flockhart DA, Skaar TC (2005) Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol 55:471–478. https://doi.org/10.1007/s00280-004-0926-7
Klein DJ, Thorn CF, Desta Z, Flockhart DA, Altman RB, Klein TE (2013) PharmGKB summary: tamoxifen pathway, pharmacokinetics. Pharmacogenet Genomics 23:643–647. https://doi.org/10.1097/FPC.0b013e3283656bc1
Singh MS, Francis PA, Michael M (2011) Tamoxifen, cytochrome P450 genes and breast cancer clinical outcomes. Breast 20:111–118. https://doi.org/10.1016/j.breast.2010.11.003
Ahmad A, Shahabuddin S, Sheikh S, Kale P, Krishnappa M, Rane RC, Ahmad I (2010) Endoxifen, a new cornerstone of breast cancer therapy: demonstration of safety, tolerability, and systemic bioavailability in healthy human subjects. Clin Pharmacol Ther 88:814–817. https://doi.org/10.1038/clpt.2010.196
Johnson MD, Zuo H, Lee KH, Trebley JP, Rae JM, Weatherman RV, Desta Z, Flockhart DA, Skaar TC (2004) Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat 85:151–159. https://doi.org/10.1023/B:BREA.0000025406.31193.e8
Wu X, Hawse JR, Subramaniam M, Goetz MP, Ingle JN, Spelsberg TC (2009) The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor alpha for degradation in breast cancer cells. Cancer Res 69:1722–1727. https://doi.org/10.1158/0008-5472.CAN-08-3933
Thompson DS, Spanier CA, Vogel VG (1999) The relationship between tamoxifen, estrogen, and depressive symptoms. Breast J 5:375–382
Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA (2006) Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat 99:215–220. https://doi.org/10.1007/s10549-006-9193-0
Perez EA (2007) Safety profiles of tamoxifen and the aromatase inhibitors in adjuvant therapy of hormone-responsive early breast cancer. Ann Oncol 18 Suppl 8:viii26–35. https://doi.org/10.1093/annonc/mdm263
Shen W, Stearns V (2009) Treatment strategies for hot flushes. Expert Opin Pharmacother 10:1133–1144. https://doi.org/10.1517/14656560902868217
Flockhart Table ™. http://medicine.iupui.edu/clinpharm/ddis/main-table
Dehal SS, Kupfer D (1997) CYP2D6 catalyzes tamoxifen 4-hydroxylation in human liver. Cancer Res 57:3402–3406
Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, Hayes DF, Desta Z, Flockhart DA (2003) Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 95:1758–1764
Jin Y, Desta Z, Stearns V, Ward B, Ho H, Lee KH, Skaar T, Storniolo AM, Li L, Araba A, Blanchard R, Nguyen A, Ullmer L, Hayden J, Lemler S, Weinshilboum RM, Rae JM, Hayes DF, Flockhart DA (2005) CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 97:30–39. https://doi.org/10.1093/jnci/dji005
Teft WA, Gong IY, Dingle B, Potvin K, Younus J, Vandenberg TA, Brackstone M, Perera FE, Choi YH, Zou G, Legan RM, Tirona RG, Kim RB (2013) CYP3A4 and seasonal variation in vitamin D status in addition to CYP2D6 contribute to therapeutic endoxifen level during tamoxifen therapy. Breast Cancer Res Treat 139:95–105. https://doi.org/10.1007/s10549-013-2511-4
Borges S, Desta Z, Li L, Skaar TC, Ward BA, Nguyen A, Jin Y, Storniolo AM, Nikoloff DM, Wu L, Hillman G, Hayes DF, Stearns V, Flockhart DA (2006) Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther 80:61–74. https://doi.org/10.1016/j.clpt.2006.03.013
Tamoxifen Product Monograph. Mylan Pharmaceuticals ULC.2014
Gaedigk A, Sangkuhl K, Whirl-Carrillo M, Klein T, Leeder JS (2016) Prediction of CYP2D6 phenotype from genotype across world populations. Genet Med 19:69–76. https://doi.org/10.1038/gim.2016.80
Welzen ME, Dezentje VO, van Schaik RH, Colbers AP, Guchelaar HJ, van Erp NP, den Hartigh J, Burger DM, van Laarhoven HW (2015) The effect of tamoxifen dose increment in patients with impaired CYP2D6 activity. Therapeutic drug monitoring 37:501–507. https://doi.org/10.1097/FTD.0000000000000195
Dezentje VO, Opdam FL, Gelderblom H, Hartigh den J, Van der Straaten T, Vree R, Maartense E, Smorenburg CH, Putter H, Dieudonne AS, Neven P, Van de Velde CJ, Nortier JW, Guchelaar HJ (2015) CYP2D6 genotype- and endoxifen-guided tamoxifen dose escalation increases endoxifen serum concentrations without increasing side effects. Breast Cancer Res Treat 153:583–590. https://doi.org/10.1007/s10549-015-3562-5
Hertz DL, Deal A, Ibrahim JG, Walko CM, Weck KE, Anderson S, Magrinat G, Olajide O, Moore S, Raab R, Carrizosa DR, Corso S, Schwartz G, Graham M, Peppercorn JM, Jones DR, Desta Z, Flockhart DA, Evans JP, McLeod HL, Carey LA, Irvin WJ Jr (2016) Tamoxifen dose escalation in patients with diminished CYP2D6 activity normalizes endoxifen concentrations without increasing toxicity. Oncologist 21:795–803. https://doi.org/10.1634/theoncologist.2015-0480
Kiyotani K, Mushiroda T, Imamura CK, Tanigawara Y, Hosono N, Kubo M, Sasa M, Nakamura Y, Zembutsu H (2012) Dose-adjustment study of tamoxifen based on CYP2D6 genotypes in Japanese breast cancer patients. Breast Cancer Res Treat 131:137–145. https://doi.org/10.1007/s10549-011-1777-7
Zhou SF (2009) Polymorphism of human cytochrome P450 2D6 and its clinical significance: part II. Clin Pharmacokinet 48:761–804. https://doi.org/10.2165/11318070-000000000-00000
Shah RR, Smith RL (2015) Addressing phenoconversion: the Achilles’ heel of personalized medicine. Br J Clin Pharmacol 79(2):222–240
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ, Group GW (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336:924–926. https://doi.org/10.1136/bmj.39489.470347.AD
Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A, Schunemann HJ, Group GW (2008) Going from evidence to recommendations. BMJ 336:1049–1051. https://doi.org/10.1136/bmj.39493.646875.AE
Crews KR, Gaedigk A, Dunnenberger HM, Klein TE, Shen DD, Callaghan JT, Kharasch ED, Skaar TC, Clinical Pharmacogenetics Implementation C (2012) Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for codeine therapy in the context of cytochrome P450 2D6 (CYP2D6) genotype. Clin Pharmacol Ther 91:321–326. https://doi.org/10.1038/clpt.2011.287
CYP2D6 Frequency Table. https://www.pharmgkb.org/page/cyp2d6RefMaterials
Province MA, Goetz MP, Brauch H et al (2014) CYP2D6 genotype and adjuvant tamoxifen: meta-analysis of heterogeneous study populations. Clin Pharmacol Ther 95:216–227. https://doi.org/10.1038/clpt.2013.186
Wright GE, Carleton B, Hayden MR, Ross CJ (2018) The global spectrum of protein-coding pharmacogenomic diversity. Pharmacogenomics J 18:187–195. https://doi.org/10.1038/tpj.2016.77
Rae JM, Regan MM, Thibert JN, Gersch C, Thomas D, Leyland-Jones B, Viale G, Pusztai L, Hayes DF, Skaar T, Van Poznak C (2013) Concordance between CYP2D6 genotypes obtained from tumor-derived and germline DNA. J Natl Cancer Inst 105:1332–1334. https://doi.org/10.1093/jnci/djt204
Gaedigk A, Ndjountche L, Divakaran K, Dianne Bradford L, Zineh I, Oberlander TF, Brousseau DC, McCarver DG, Johnson JA, Alander SW, Wayne Riggs K, Steven Leeder J (2007) Cytochrome P4502D6 (CYP2D6) gene locus heterogeneity: characterization of gene duplication events. Clin Pharmacol Ther 81:242–251. https://doi.org/10.1038/sj.clpt.6100033
Goetz MP, Sun JX, Suman VJ, Silva GO, Perou CM, Nakamura Y, Cox NJ, Stephens PJ, Miller VA, Ross JS, Chen D, Safgren SL, Kuffel MJ, Ames MM, Kalari KR, Gomez HL, Gonzalez-Angulo AM, Burgues O, Brauch HB, Ingle JN, Ratain MJ, Yelensky R (2014) Loss of heterozygosity at the CYP2D6 locus in breast cancer: implications for germline pharmacogenetic studies. J Natl Cancer Inst 107. https://doi.org/10.1093/jnci/dju401
Castells A, Gusella JF, Ramesh V, Rustgi AK (2000) A region of deletion on chromosome 22q13 is common to human breast and colorectal cancers. Cancer Res 60:2836–2839
Regan MM, Leyland-Jones B, Bouzyk M, Pagani O, Tang W, Kammler R, Dell’orto P, Biasi MO, Thurlimann B, Lyng MB, Ditzel HJ, Neven P, Debled M, Maibach R, Price KN, Gelber RD, Coates AS, Goldhirsch A, Rae JM, Viale G, Breast International Group 1–98 Collaborative G (2012) CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the breast international group 1–98 trial. J Natl Cancer Inst 104:441–451. https://doi.org/10.1093/jnci/djs125
Stanton V Jr (2012) Re: CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the Breast International Group 1–98 trial. J Natl Cancer Inst 104:1265–1266. https://doi.org/10.1093/jnci/djs305. author reply 1266–1268.
Nakamura Y, Ratain MJ, Cox NJ, McLeod HL, Kroetz DL, Flockhart DA (2012) Re: CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the Breast International Group 1–98 trial. J Natl Cancer Inst 104:1264. https://doi.org/10.1093/jnci/djs304. author reply 1266–1268.
Goetz MP, Ingle JN (2014) CYP2D6 genotype and tamoxifen: considerations for proper nonprospective studies. Clin Pharmacol Ther 96:141–144. https://doi.org/10.1038/clpt.2014.99
Zeng Z, Liu Y, Liu Z, You J, Chen Z, Wang J, Peng Q, Xie L, Li R, Li S, Qin X (2013) CYP2D6 polymorphisms influence tamoxifen treatment outcomes in breast cancer patients: a meta-analysis. Cancer Chemother Pharmacol 72:287–303. https://doi.org/10.1007/s00280-013-2195-9
Kiyotani K, Mushiroda T, Hosono N, Tsunoda T, Kubo M, Aki F, Okazaki Y, Hirata K, Takatsuka Y, Okazaki M, Ohsumi S, Yamakawa T, Sasa M, Nakamura Y, Zembutsu H (2010) Lessons for pharmacogenomics studies: association study between CYP2D6 genotype and tamoxifen response. Pharmacogenet Genomics 20:565–568. https://doi.org/10.1097/FPC.0b013e32833af231
Henry NL, Hayes DF, Rae JM (2009) CYP2D6 testing for breast cancer patients: is there more to the story? Oncology (Williston Park) 23(1236):1243, 1249
Goetz MP, Suman VJ, Hoskin TL, Gnant M, Filipits M, Safgren SL, Kuffel M, Jakesz R, Rudas M, Greil R, Dietze O, Lang A, Offner F, Reynolds CA, Weinshilboum RM, Ames MM, Ingle JN (2013) CYP2D6 metabolism and patient outcome in the Austrian Breast and Colorectal Cancer Study Group trial (ABCSG) 8. Clin Cancer Res 19:500–507. https://doi.org/10.1158/1078-0432.CCR-12-2153
Schroth W, Goetz MP, Hamann U, Fasching PA, Schmidt M, Winter S, Fritz P, Simon W, Suman VJ, Ames MM et al (2009) Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA 302(13):1429–1436
Lammers LA, Mathijssen RH, van Gelder T, Bijl MJ, de Graan AJ, Seynaeve C, van Fessem MA, Berns EM, Vulto AG, van Schaik RH (2010) The impact of CYP2D6-predicted phenotype on tamoxifen treatment outcome in patients with metastatic breast cancer. Br J Cancer 103:765–771. https://doi.org/10.1038/sj.bjc.6605800
Lim HS, Ju Lee H, Seok Lee K, Sook Lee E, Jang IJ, Ro J (2007) Clinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancer. J Clin Oncol 25:3837–3845. https://doi.org/10.1200/JCO.2007.11.4850
Visvanathan K, Hurley P, Bantug E, Brown P, Col NF, Cuzick J, Davidson NE, Decensi A, Fabian C, Ford L, Garber J, Katapodi M, Kramer B, Morrow M, Parker B, Runowicz C, Vogel VG III, Wade JL, Lippman SM (2013) Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 31:2942–2962. https://doi.org/10.1200/JCO.2013.49.3122
Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Solky AJ, Stearns V, Winer EP, Griggs JJ (2016) Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline update on ovarian suppression. J Clin Oncol 34:1689–1701. https://doi.org/10.1200/JCO.2015.65.9573
Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS, Group AT (2005) Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365:60–62. https://doi.org/10.1016/S0140-6736(04)17666-6
Early Breast Cancer Trialists’ Collaborative Group (2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386:1341–1352. https://doi.org/10.1016/S0140-6736(15)61074-1
De Placido S, Gallo C, De Laurentiis M, Bisagni G, Arpino G, Sarobba MG, Riccardi F, Russo A, Del Mastro L, Cogoni AA, Cognetti F, Gori S, Foglietta J, Frassoldati A, Amoroso D, Laudadio L, Moscetti L, Montemurro F, Verusio C, Bernardo A, Lorusso V, Gravina A, Moretti G, Lauria R, Lai A, Mocerino C, Rizzo S, Nuzzo F, Carlini P, Perrone F, Investigators GIM (2018) Adjuvant anastrozole versus exemestane versus letrozole, upfront or after 2 years of tamoxifen, in endocrine-sensitive breast cancer (FATA-GIM3): a randomised, phase 3 trial. Lancet Oncol 19:474–485. https://doi.org/10.1016/S1470-2045(18)30116-5
Miller WR (2003) Aromatase inhibitors: mechanism of action and role in the treatment of breast cancer. Semin Oncol 30:3–11
Kanis JA, McCloskey EV, Powles T, Paterson AH, Ashley S, Spector T (1999) A high incidence of vertebral fracture in women with breast cancer. Br J Cancer 79:1179–1181. https://doi.org/10.1038/sj.bjc.6690188
Lester J, Dodwell D, McCloskey E, Coleman R (2005) The causes and treatment of bone loss associated with carcinoma of the breast. Cancer Treat Rev 31:115–142. https://doi.org/10.1016/j.ctrv.2005.01.008
Ramaswamy B, Shapiro CL (2003) Osteopenia and osteoporosis in women with breast cancer. Semin Oncol 30:763–775
Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S (1996) Effect of tamoxifen on bone mineral density measured by dual-energy X-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol 14:78–84
Love RR, Mazess RB, Barden HS, Epstein S, Newcomb PA, Jordan VC, Carbone PP, DeMets DL (1992) Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med 326:852–856. https://doi.org/10.1056/NEJM199203263261302
Kristensen B, Ejlertsen B, Dalgaard P, Larsen L, Holmegaard SN, Transbol I, Mouridsen HT (1994) Tamoxifen and bone metabolism in postmenopausal low-risk breast cancer patients: a randomized study. J Clin Oncol 12:992–997
Ganz PA, Cecchini RS, Julian TB, Margolese RG, Costantino JP, Vallow LA, Albain KS, Whitworth PW, Cianfrocca ME, Brufsky AM, Gross HM, Soori GS, Hopkins JO, Fehrenbacher L, Sturtz K, Wozniak TF, Seay TE, Mamounas EP, Wolmark N (2016) Patient-reported outcomes with anastrozole versus tamoxifen for postmenopausal patients with ductal carcinoma in situ treated with lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, phase 3 clinical trial. Lancet 387:857–865. https://doi.org/10.1016/S0140-6736(15)01169-1
Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS (2008) The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther 83:234–242. https://doi.org/10.1038/sj.clpt.6100406
Gong IY, Teft WA, Ly J, Chen YH, Alicke B, Kim RB, Choo EF (2013) Determination of clinically therapeutic endoxifen concentrations based on efficacy from human MCF7 breast cancer xenografts. Breast Cancer Res Treat 139:61–69. https://doi.org/10.1007/s10549-013-2530-1
Saladores P, Murdter T, Eccles D, Chowbay B, Zgheib NK, Winter S, Ganchev B, Eccles B, Gerty S, Tfayli A, Lim JS, Yap YS, Ng RC, Wong NS, Dent R, Habbal MZ, Schaeffeler E, Eichelbaum M, Schroth W, Schwab M, Brauch H (2015) Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharmacogenomics J 15:84–94. https://doi.org/10.1038/tpj.2014.34
Madlensky L, Natarajan L, Tchu S, Pu M, Mortimer J, Flatt SW, Nikoloff DM, Hillman G, Fontecha MR, Lawrence HJ, Parker BA, Wu AH, Pierce JP (2011) Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther 89:718–725. https://doi.org/10.1038/clpt.2011.32
Zembutsu H, Nakamura S, Akashi ST, Kuwayama T, Watanabe C, Takamaru T, Takei H, Ishikawa T, Miyahara K, Matsumoto H, Hasegawa Y, Kutomi G, Shima H, Satomi F, Okazaki M, Zaha H, Onomura M, Matsukata A, Sagara Y, Baba S, Yamada A, Shimada K, Shimizu D, Tsugawa K, Shimo A, Tan EY, Hartman M, Chan CW, Lee SC, Nakamura Y (2016) Significant effect of polymorphisms in CYP2D6 on response to tamoxifen therapy for breast cancer; a prospective multicenter study. Clin Cancer Res Clin Cancer Res 23:2019–2026. https://doi.org/10.1158/1078-0432.CCR-16-1779
Goetz MP, Sangkuhl K, Guchelaar HJ, Schwab M, Province M, Whirl-Carrillo M, Symmans WF, McLeod HL, Ratain MJ, Zembutsu H, Gaedigk A, van Schaik RH, Ingle JN, Caudle KE, Klein TE (2018) Clinical pharmacogenetics implementation consortium (CPIC) guideline for CYP2D6 and tamoxifen therapy. Clin Pharmacol Ther 103:770–777. https://doi.org/10.1002/cpt.1007
Binkhorst L, Mathijssen RH, Jager A, van Gelder T (2015) Individualization of tamoxifen therapy: much more than just CYP2D6 genotyping. Cancer Treat Rev 41:289–299. https://doi.org/10.1016/j.ctrv.2015.01.002
de Vries Schultink AHM, Huitema ADR, Beijnen JH (2018) Therapeutic Drug Monitoring of endoxifen as an alternative for CYP2D6 genotyping in individualizing tamoxifen therapy. Breast 42:38–40
Maximov PY, McDaniel RE, Fernandes DJ, Korostyshevskiy VR, Bhatta P, Murdter TE, Flockhart DA, Jordan VC (2014) Simulation with cells in vitro of tamoxifen treatment in premenopausal breast cancer patients with different CYP2D6 genotypes. Br J Pharmacol 171:5624–5635. https://doi.org/10.1111/bph.12864
Maximov PY, McDaniel RE, Fernandes DJ, Bhatta P, Korostyshevskiy VR, Curpan RF, Jordan VC (2014) Pharmacological relevance of endoxifen in a laboratory simulation of breast cancer in postmenopausal patients. J Natl Cancer Inst 106. https://doi.org/10.1093/jnci/dju283
Drogemoller BI, Wright GE, Niehaus DJ, Emsley R, Warnich L (2013) Next-generation sequencing of pharmacogenes: a critical analysis focusing on schizophrenia treatment. Pharmacogenetics Genomics 23(12):666–674
Whirl-Carrillo M, McDonagh EM, Hebert JM, Gong L, Sangkuhl K, Thorn CF, Altman RB, Klein TE (2012) Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther 92(4):414–417
Acknowledgements
The members of the Canadian Pharmacogenomics Network for Drug Safety Clinical Recommendations Group are: Vancouver, BC, Canada—University of British Columbia: Ursula Amstutz, Bruce C. Carleton, Wan C. Chang, Mary B. Connolly, Francois Dionne, Britt I. Drögemöller, Karen A. Gelmon, Gabriella Groeneweg, Catrina M. Loucks, Stuart M. MacLeod, Sheila Pritchard (Sheilapritchard@me.com), Shahrad R. Rassekh, Colin J.D. Ross, Shubhayan Sanatani, Joanne Shih, Reo Tanoshima, Sean A. Virani, Galen E.B. Wright. Calgary AB, Canada—University of Calgary: José G. Monzon. Edmonton AB, Canada—University of Alberta: Amit P. Bhavsar. London, ON, Canada—University of Western Ontario and London Health Sciences Centre: Michael J. Rieder. Toronto, ON, Canada—Sunnybrook Health Sciences Centre: Neil H. Shear; University of Toronto and Hospital for Sick Children: Shinya Ito, Ontario Cancer Institute: Geoffrey Liu. Montréal, QC, Canada—Philip Khayat. Stanford, CA, USA—Stanford University: Daniel Bernstein. Orlando, FL, USA—University of Florida: Lawrence J. Lesko. Singapore—Agency for Science, Technology and Research—Folefac Aminkeng.
Funding
This study was funded by Canadian Institutes of Health (CIHR) Research Meetings, Planning, and Dissemination Grant–Knowledge Translation Supplement (FRN 114403). BID received stipends from the CIHR, CIHR-DSECT and the Michael Smith Foundation for Health Research. GEBW received stipends from the CIHR and CIHR-DSECT.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
The members of the CPNDS Clinical Recommendations Group were listed in acknowlegements.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Drögemöller, B.I., Wright, G.E.B., Shih, J. et al. CYP2D6 as a treatment decision aid for ER-positive non-metastatic breast cancer patients: a systematic review with accompanying clinical practice guidelines. Breast Cancer Res Treat 173, 521–532 (2019). https://doi.org/10.1007/s10549-018-5027-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-5027-0