Abstract
Purpose
This is the largest study to date evaluating response rates and pathologic complete response (pCR) and predictors thereof, based on molecular subtype, in women with breast cancer having undergone neoadjuvant chemotherapy (NC).
Methods
The National Cancer Database was queried for women with cT1-4N1-3M0 breast cancer having received NC. Patients were divided into four subtypes: luminal A, luminal B, Her2, or triple negative (TN). Multivariable logistic regression ascertained factors associated with developing pCR. Kaplan–Meier analysis evaluated overall survival (OS) between patients by degree of response to NC when stratifying patients by subtype.
Results
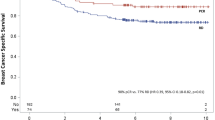
Of a total of 13,939 women, 322 (2%) were luminal A, 5941 (43%) luminal B, 2274 (16%) Her2, and 5402 (39%) TN. Overall, 19% of all patients achieved pCR, the lowest in luminal A (0.3%) and the highest in Her2 (38.7%). Molecular subtype was an independent predictor of both pCR and OS in this population. Clinical downstaging was associated with improved survival, mostly in women with luminal B, Her2, and TN subtypes. Subgroup analysis of the pCR population demonstrated 5-year OS in the luminal B, Her2, and TN cohorts of 93.0, 94.2, and 90.6%, respectively (Her2 vs. TN, p = 0.016).
Conclusions
Assessing nearly 14,000 women from a contemporary United States database, this is the largest known study examining the relationship between response to NC and molecular subtype. Women with luminal A disease are the least likely to undergo pCR, with the highest rates in Her2 disease. Degree of response is associated with OS, especially in luminal B, Her2, and TN patients. Despite the comparatively higher likelihood of achieving pCR in TN cases, this subgroup may still experience a survival detriment, which has implications for an ongoing national randomized trial.



Similar content being viewed by others
References
Gao JJ, Swain SM (2018) Luminal A breast cancer and molecular assays: a review. Oncologist. https://doi.org/10.1634/theoncologist.2017-0535
Mamounas EP, Anderson SJ, Dignam JJ et al (2012) Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of national surgical adjuvant breast and bowel project B-18 and B-27. J Clin Oncol 30:3960–3966
Mamounas EP, Bandos H, White JR et al. NRG oncology/NSABP B-51/RTOG 1304: Phase III trial to determine if chest wall and regional nodal radiotherapy (CWRNRT) post mastectomy (Mx) or the addition of RNRT to breast RT post breast-conserving surgery (BCS) reduces invasive breast cancer recurrence free interval (IBCRFI) in patients (pts) with positive axillary (PAx) nodes who are ypN0 after neoadjuvant chemotherapy (NC). J Clin Oncol 2017. https://doi.org/10.1200/JCO.2017.35.15_suppl.TPS589
Carey LA, Dees EC, Sawyer L et al (2007) The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 13:2329–2334
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384:164–172
Bilimoria KY, Stewart AK, Winchester DP et al (2008) The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 15:683–690
National Comprehensive Cancer Network (2018) Breast Cancer. Version 1. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 26 Mar 2018
Hansen EK, Roach M III (eds) (2010) Handbook of evidence-based radiation oncology. 2nd edn. Springer, New York
DeVita VT Jr, Lawrence TS, Rosenberg SA (eds) (2011) DeVita, Hellman, and Rosenberg’s cancer: principles and practice of oncology, 9th edn. Lippincott Williams & Wilkins, Philadelphia
Lv M, Li B, Mao X, Yao F, Jin F (2011) Predictive role of molecular subtypes in response to neoadjuvant chemotherapy in breast cancer patients in Northeast China. Asian Pac J Cancer Prev 12:2411–2417
Harbeck N, Thomssen C, Gnant M (2013) St. Gallen 2013: brief preliminary summary of the consensus discussion. Breast Care (Basel) 8:102–109
Breastcancer.org. Molecular subtypes of breast cancer. http://www.breastcancer.org/symptoms/types/molecular-subtypes. Accessed 26 Mar 2018
Mazouni C, Peintinger F, Wan-Kau S et al (2007) Residual ductal carcinoma in situ in patients with complete eradication of invasive breast cancer after neoadjuvant chemotherapy does not adversely affect patient outcome. J Clin Oncol 25:2650–2655
Liedtke C, Mazouni C, Hess KR et al (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26:1275–1281
Haque W, Verma V, Butler EB, Teh BS (2017) Patterns of care and outcomes of multi-agent versus single-agent chemotherapy as part of multimodal management of low grade glioma. J Neurooncol 133:369–375
Haque W, Verma V, Butler EB, Teh BS (2017) National practice patterns and outcomes for T4b urothelial cancer of the bladder. Clin Genitourin Cancer. https://doi.org/10.1016/j.clgc.2017.08.013
Haque W, Verma V, Fakhreddine M et al (2017) Addition of chemotherapy to definitive radiotherapy for IB1 and IIA1 cervical cancer: analysis of the National Cancer Data Base. Gynecol Oncol 144:28–33
Haque W, Verma V, Butler EB, Teh BS (2017) Radical cystectomy versus chemoradiation for musle-invasive bladder cancer: impact of treatment facility and sociodemographics. Anticancer Res 37:5603–5608
Haque W, Verma V, Butler EB, Teh BS (2017) Radiation dose in neoadjuvant chemoradiation therapy for esophageal cancer: patterns of care and outcomes from the National Cancer Data Base. J Gastrointest Oncol. https://doi.org/10.21037/jgo.2017.09.12
Haque W, Verma V, Butler EB, Teh BS (2017) Addition of chemotherapy to hypofractionated radiotherapy for glioblastoma: practice patterns, outcomes, and predictors of survival. J Neurooncol. https://doi.org/10.1007/s11060-017-2654-y
Haque W, Verma V, Butler EB, Teh BS (2017) Chemotherapy versus chemoradiation for node-positive bladder cancer: practice patterns and outcomes from the National Cancer Data Base. Bladder Cancer 3:283–291
Haque W, Verma V, Butler EB, Teh BS (2017) Definitive chemoradiation at high volume facilities is associated with improved survival in glioblastoma. J Neurooncol. https://doi.org/10.1007/s11060-017-2563-0
Haque W, Verma V, Bernicker E, Butler EB, Teh BS (2017) Management of pathologic node-positive disease following initial surgery for clinical T1-2 N0 esophageal cancer: patterns of care and outcomes from the national cancer data base. Acta Oncol. https://doi.org/10.1080/0284186X.2017.1409435
Haque W, Lewis GD, Verma V, Darcourt JG, Butler EB, Teh BS (2017) The role of adjuvant chemotherapy in locally advanced bladder cancer. Acta Oncol. https://doi.org/10.1080/0284186X.2017.1415461
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Haque, W., Verma, V., Hatch, S. et al. Response rates and pathologic complete response by breast cancer molecular subtype following neoadjuvant chemotherapy. Breast Cancer Res Treat 170, 559–567 (2018). https://doi.org/10.1007/s10549-018-4801-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4801-3