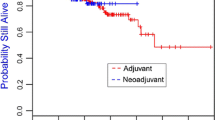
Abstract
Purpose
BRCA1/2 mutations influence the molecular characteristics and the effects of systemic treatment of breast cancer. This study investigates the impact of germline BRCA1/2 mutations on pathological complete response and prognosis in patients receiving neoadjuvant systemic chemotherapy.
Methods
Breast cancer patients were tested for a BRCA1/2 mutation in clinical routine work and were treated with anthracycline-based or platinum-based neoadjuvant chemotherapy between 1997 and 2015. These patients were identified in the tumor registry of the Breast Center of the University of Erlangen (Germany). Logistic regression and Cox regression analyses were performed to investigate the associations between BRCA1/2 mutation status, pathological complete response, disease-free survival, and overall survival.
Results
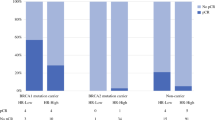
Among 355 patients, 59 had a mutation in BRCA1 or in BRCA2 (16.6%), 43 in BRCA1 (12.1%), and 16 in BRCA2 (4.5%). Pathological complete response defined as “ypT0; ypN0” was observed in 54.3% of BRCA1/2 mutation carriers, but only in 22.6% of non-carriers. The adjusted odds ratio was 2.48 (95% CI 1.26–4.91) for BRCA1/2 carriers versus non-carriers. Patients who achieved a pathological complete response had better disease-free survival and overall survival rates compared with those who did not achieve a pathological complete response, regardless of BRCA1/2 mutation status.
Conclusions
BRCA1/2 mutation status leads to better responses to neoadjuvant chemotherapy in breast cancer. Pathological complete response is the main predictor of disease-free survival and overall survival, independently of BRCA1/2 mutation status.



Similar content being viewed by others
References
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172
von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J et al (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30(15):1796–1804
Cortesi L, Masini C, Cirilli C, Medici V, Marchi I, Cavazzini G, Pasini G, Turchetti D, Federico M (2010) Favourable ten-year overall survival in a Caucasian population with high probability of hereditary breast cancer. BMC Cancer 10:90
Arun B, Bayraktar S, Liu DD, Gutierrez Barrera AM, Atchley D, Pusztai L, Litton JK, Valero V, Meric-Bernstam F, Hortobagyi GN et al (2011) Response to neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers: a single-institution experience. J Clin Oncol 29(28):3739–3746
Rennert G, Bisland-Naggan S, Barnett-Griness O, Bar-Joseph N, Zhang S, Rennert HS, Narod SA (2007) Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. New Engl J Med 357(2):115–123
Copson ER, Maishman TC, Tapper WJ, Cutress RI, Greville-Heygate S, Altman DG, Eccles B, Gerty S, Durcan LT, Jones L et al (2018) Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol 19(2):169–180
Zhong Q, Peng HL, Zhao X, Zhang L, Hwang WT (2015) Effects of BRCA1- and BRCA2-related mutations on ovarian and breast cancer survival: a meta-analysis. Clin Cancer Res 21(1):211–220
Goodwin PJ, Phillips KA, West DW, Ennis M, Hopper JL, John EM, O’Malley FP, Milne RL, Andrulis IL, Friedlander ML et al (2012) Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an International Prospective Breast Cancer Family Registry population-based cohort study. J Clin Oncol 30(1):19–26
Huzarski T, Byrski T, Gronwald J, Gorski B, Domagala P, Cybulski C, Oszurek O, Szwiec M, Gugala K, Stawicka M et al (2013) Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J Clin Oncol 31(26):3191–3196
van den Broek AJ, Schmidt MK, van ‘t Veer LJ, Tollenaar RA, van Leeuwen FE (2015) Worse breast cancer prognosis of BRCA1/BRCA2 mutation carriers: what’s the evidence? A systematic review with meta-analysis. PLoS ONE 10(3):e0120189
Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M, Mierzwa T, Szwiec M, Wisniowski R, Siolek M et al (2010) Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol 28(3):375–379
Byrski T, Huzarski T, Dent R, Gronwald J, Zuziak D, Cybulski C, Kladny J, Gorski B, Lubinski J, Narod SA (2009) Response to neoadjuvant therapy with cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat 115(2):359–363
Byrski T, Huzarski T, Dent R, Marczyk E, Jasiowka M, Gronwald J, Jakubowicz J, Cybulski C, Wisniowski R, Godlewski D et al (2014) Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat 147(2):401–405
Paluch-Shimon S, Friedman E, Berger R, Papa M, Dadiani M, Friedman N, Shabtai M, Zippel D, Gutman M, Golan T et al (2016) Neo-adjuvant doxorubicin and cyclophosphamide followed by paclitaxel in triple-negative breast cancer among BRCA1 mutation carriers and non-carriers. Breast Cancer Res Treat 157(1):157–165
Beckmann MW, Brucker C, Hanf V, Rauh C, Bani MR, Knob S, Petsch S, Schick S, Fasching PA, Hartmann A et al (2011) Quality assured health care in certified breast centers and improvement of the prognosis of breast cancer patients. Onkologie 34(7):362–367
Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ (2009) Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol 20(8):1319–1329
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M et al (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med 134(6):907–922
Fasching PA, Heusinger K, Haberle L, Niklos M, Hein A, Bayer CM, Rauh C, Schulz-Wendtland R, Bani MR, Schrauder M et al (2011) Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer 11:486
Meindl A, Ditsch N, Kast K, Rhiem K, Schmutzler RK (2011) Hereditary breast and ovarian cancer: new genes, new treatments, new concepts. Dtsch Arztebl Int 108(19):323–330
Li H (2014) Toward better understanding of artifacts in variant calling from high-coverage samples. Bioinformatics 30(20):2843–2851
Szabo C, Masiello A, Ryan JF, Brody LC (2000) The breast cancer information core: database design, structure, and scope. Hum Mutat 16(2):123–131
Haberle L, Fasching PA, Brehm B, Heusinger K, Jud SM, Loehberg CR, Hack CC, Preuss C, Lux MP, Hartmann A et al (2016) Mammographic density is the main correlate of tumors detected on ultrasound but not on mammography. Int J Cancer 139(9):1967–1974
Burghaus S, Haberle L, Schrauder MG, Heusinger K, Thiel FC, Hein A, Wachter D, Strehl J, Hartmann A, Ekici AB et al (2015) Endometriosis as a risk factor for ovarian or endometrial cancer - results of a hospital-based case-control study. BMC Cancer 15:751
Salmen J, Neugebauer J, Fasching PA, Haeberle L, Huober J, Wockel A, Rauh C, Schuetz F, Weissenbacher T, Kost B et al (2014) Pooled analysis of the prognostic relevance of progesterone receptor status in five German cohort studies. Breast Cancer Res Treat 148(1):143–151
Hahnen E, Lederer B, Hauke J, Loibl S, Krober S, Schneeweiss A, Denkert C, Fasching PA, Blohmer JU, Jackisch C et al (2017) Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: Secondary analysis of the GeparSixto randomized clinical trial. JAMA Oncol 3(10):1378–1385
Fasching PA, Loibl S, Eidtmann H, Tesch H, Untch M, Hilfrich J, Schem C, Rezai M, Gerber B, Costa SD et al. (2016) BRCA mutations, therapy response and prognosis in the neoadjuvant GeparQuinto study. AACR Cancer Res 76 (4 Suppl): S5-06
Lux MP, Janni W, Hartkopf AD, Nabieva N, Taran FA, Overkamp F, Kolberg HC, Hadji P, Tesch H, Ettl J et al (2017) Update breast cancer 2017—implementation of novel therapies. Geburtshilfe Frauenheilkd 77(12):1281–1290
Fasching PA, Blohmer JU, Burchardi N, Costa SD, Denkert C, Hanusch C, Huober JB, Von Minckwitz G, Paepke S, Schneeweiss A et al. (2016) A randomized phase II trial to assess the efficacy of paclitaxel and olaparib in comparison to paclitaxel/carboplatin followed by epirubicin/cyclophosphamide as neoadjuvant chemotherapy in patients with HER2-negative early breast cancer and homologous recombination deficiency (HRD). GeparOLA. J Clin Oncol 34(15 Suppl):TPS1096
Schneeweiss A, Jackisch C, Schmatloch S, Aktas B, Denkert C, Schem C, Wiebringhaus H, Kümmel S, Rhiem K, Warm M et al. (2018) Survival analysis of the prospectively randomized phase III GeparSepto trial comparing neoadjuvant chemotherapy with weekly nab-paclitaxel with solvent-based paclitaxel followed by anthracycline–cyclophosphamide for patients with early breast cancer—GBG69. In: Proceedings of the 2017 San Antonio Breast Cancer Symposium, AACR, Dec 5–9 2017, San Antonio, TX
von Minckwitz G, Blohmer JU, Costa SD, Denkert C, Eidtmann H, Eiermann W, Gerber B, Hanusch C, Hilfrich J, Huober J et al (2013) Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol 31(29):3623–3630
von Minckwitz G, Hahnen E, Fasching PA, Hauke J, Schneeweiss A, Salat C, Rezai M, Blohmer JU, Zahm DM, Jackisch C (2014) Pathological complete response (pCR) rates after carboplatin-containing neoadjuvant chemotherapy in patients with germline BRCA (g BRCA) mutation and triple-negative breast cancer (TNBC): results from GeparSixto. Int J Clin Oncol 32:5s
Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C et al (2005) Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434(7035):917–921
Mavaddat N, Barrowdale D, Andrulis IL, Domchek SM, Eccles D, Nevanlinna H, Ramus SJ, Spurdle A, Robson M, Sherman M et al (2012) Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev 21(1):134–147
Timms KM, Abkevich V, Hughes E, Neff C, Reid J, Morris B, Kalva S, Potter J, Tran TV, Chen J et al (2014) Association of BRCA1/2 defects with genomic scores predictive of DNA damage repair deficiency among breast cancer subtypes. Breast Cancer Res 16(6):475
Zhang J, Powell SN (2005) The role of the BRCA1 tumor suppressor in DNA double-strand break repair. Mol Cancer Res 3(10):531–539
Narod SA, Huzarski T, Gronwald J, Byrski T, Marczyk E, Cybulski C, Szwiec M, Wisniowski R, Birkenfeld B, Kilar E et al. (2017) Predictors of survival for breast cancer patients with a BRCA1 mutation. Breast Cancer Res Treat 168(2), 513–521
Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q, Juul N, Leong CO, Calogrias D, Buraimoh A et al (2010) Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol 28(7):1145–1153
Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, Kuzma CS, Pluard TJ, Somlo G, Port ER et al (2015) Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 33(1):13–21
von Minckwitz G, Timms K, Untch M, Elkin EP, Hahnen E, Fasching PA, Schneeweiss A, Salat CT, Rezai M, Blohmer J-U et al (2017) Homologous repair deficiency (HRD) as measure to predict the effect of carboplatin on survival in the neoadjuvant phase II trial GeparSixto in triple-negative early breast cancer. AACR Cancer Res 77 (4 Suppl):P1-09-02
Gluz O, Nitz U, Liedtke C, Christgen M, Grischke EM, Forstbauer H, Braun M, Warm M, Hackmann J, Uleer C et al (2017) Comparison of neoadjuvant Nab-Paclitaxel + carboplatin vs nab-paclitaxel + gemcitabine in triple-negative breast cancer: randomized WSG-ADAPT-TN trial results. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djx258
von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, Blohmer JU, Jackisch C, Paepke S, Gerber B et al (2014) Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 15(7):747–756
Von Minckwitz G, Loibl S, Schneeweiss A (2015) Early survival analysis of the randomized phase II trial investigating the addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer (GeparSixto). In: San Antonio Breast Cancer Symposium, San Antonio, Dec 9 2015
Curigliano G, Burstein HJ, E PW, Gnant M, Dubsky P, Loibl S, Colleoni M, Regan MM, Piccart-Gebhart M, Senn HJ et al. (2017) De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the primary therapy of early breast cancer 2017. Ann Oncol 28(8):1700–1712
Hurvitz SA, Martin M, Symmans WF, Jung KH, Huang CS, Thompson AM, Harbeck N, Valero V, Stroyakovskiy D, Wildiers H et al (2018) Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 19(1):115–126
Acknowledgements
The authors are grateful to Michael Robertson for professional medical editing services.
Author information
Authors and Affiliations
Contributions
MW, AH, PG, and PAF contributed substantially to the acquisition and interpretation of data, to the conception and drafting of the manuscript, and to critical revision. LH performed statistical analyses and contributed to the conception, drafting, and critical revision of the manuscript. The contribution of VMF to this publication was made in partial fulfillment of the requirements for obtaining the degree of Doctor of Medicine; parts of the research published here were used for her doctoral thesis at the Medical Faculty of Friedrich Alexander University Erlangen–Nuremberg (FAU). CR, MRB, CCH, MGS, SMJ, JE, RE, ABE, JH, GV, CK, AR, AH, MPL, MWB, and AH were involved in the acquisition of patient and tumor data and genetic information. All authors have read the manuscript and have given their final approval for publication of this study.
Corresponding author
Ethics declarations
Conflict of interest
PAF has received honoraria from Amgen, Celgene, Roche, Pfizer, and Novartis. MPL has received honoraria from MSD and AstraZeneca. PG has received honoraria from Novartis and financial support for symposia from Roche, Novartis, and PharmaMar. All other authors declare that they do not have any conflicts of interest.
Ethical approval
This retrospective study and the anonymized scientific use of the data were approved by the Ethics Committee of the Medical Faculty of Friedrich Alexander University Erlangen–Nuremberg.
Informed consent
Informed consent was obtained from each individual participant included in the study.
Rights and permissions
About this article
Cite this article
Wunderle, M., Gass, P., Häberle, L. et al. BRCA mutations and their influence on pathological complete response and prognosis in a clinical cohort of neoadjuvantly treated breast cancer patients. Breast Cancer Res Treat 171, 85–94 (2018). https://doi.org/10.1007/s10549-018-4797-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4797-8