Abstract
Purpose
Male breast cancer (MBC) is a rare cancer entity, with mutations in BRCA1 and BRCA2 genes accounting for ~ 10% of patients. Multiple-gene sequencing has already entered clinical practice for female breast cancer, whereas the performance of panel testing in MBC has not been studied extensively. Therefore, the aim of this study was to evaluate the clinical utility of panel testing for MBC, by the largest gene panel used so far, through investigation of patients deriving from a population with known founder effects.
Methods
Genomic DNA from one hundred and two Greek MBC patients, unselected for age and family history, was used to prepare libraries which capture the entire coding regions of 94 cancer genes.
Results
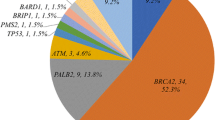
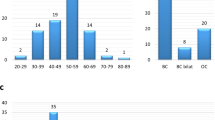
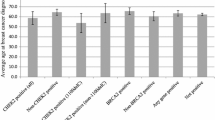
Loss-of-function (LoF) mutations were found in 12.7% of the cases, distributed in six genes: BRCA2, ATM, BRCA1, CHEK2, PMS2, and FANCL. BRCA2 mutations were the most frequent, followed by ATM mutations, accounting for 6.9 and 2%, respectively, while mutations in other genes were detected in single cases. Age at diagnosis or family history was not predictive of mutation status. Beyond mutations in established breast cancer predisposing genes, LoF mutations in PMS2 and FANCL among MBC patients are reported here for the first time.
Conclusions
Our findings, using the largest gene panel for MBC patients so far, indicate that BRCA testing should be the primary concern for MBC patients. Until sufficient evidence arises from larger studies, multiple-gene panels may be of limited benefit for MBC and their families, at least for MBC patients of specific descent.


Similar content being viewed by others
References
Korde LA, Zujewski JA, Kamin L, Giordano S, Domchek S, Anderson WF, Bartlett JM, Gelmon K, Nahleh Z, Bergh J, Cutuli B, Pruneri G, McCaskill-Stevens W, Gralow J, Hortobagyi G, Cardoso F (2010) Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol 28(12):2114–2122. https://doi.org/10.1200/JCO.2009.25.5729
Weiss JR, Moysich KB, Swede H (2005) Epidemiology of male breast cancer. Cancer Epidemiol Biomark Prev 14(1):20–26
Cardoso F, Bartlett JMS, Slaets L, van Deurzen CHM, van Leeuwen-Stok E, Porter P, Linderholm B, Hedenfalk I, Schroder C, Martens J, Bayani J, van Asperen C, Murray M, Hudis C, Middleton L, Vermeij J, Punie K, Fraser J, Nowaczyk M, Rubio IT, Aebi S, Kelly C, Ruddy KJ, Winer E, Nilsson C, Dal Lago L, Korde L, Benstead K, Bogler O, Goulioti T, Peric A, Litiere S, Aalders KC, Poncet C, Tryfonidis K, Giordano SH (2017) Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncol. https://doi.org/10.1093/annonc/mdx651
Brinton LA, Richesson DA, Gierach GL, Lacey JV Jr, Park Y, Hollenbeck AR, Schatzkin A (2008) Prospective evaluation of risk factors for male breast cancer. J Natl Cancer Inst 100(20):1477–1481. https://doi.org/10.1093/jnci/djn329
Tai YC, Domchek S, Parmigiani G, Chen S (2007) Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst 99(23):1811–1814. https://doi.org/10.1093/jnci/djm203
Breast Cancer Linkage C (1999) Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst 91(15):1310–1316
Meijers-Heijboer H, van den Ouweland A, Klijn J, Wasielewski M, de Snoo A, Oldenburg R, Hollestelle A, Houben M, Crepin E, van Veghel-Plandsoen M, Elstrodt F, van Duijn C, Bartels C, Meijers C, Schutte M, McGuffog L, Thompson D, Easton D, Sodha N, Seal S, Barfoot R, Mangion J, Chang-Claude J, Eccles D, Eeles R, Evans DG, Houlston R, Murday V, Narod S, Peretz T, Peto J, Phelan C, Zhang HX, Szabo C, Devilee P, Goldgar D, Futreal PA, Nathanson KL, Weber B, Rahman N, Stratton MR, Consortium CH-BC (2002) Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet 31(1):55–59. https://doi.org/10.1038/ng879
Wasielewski M, den Bakker MA, van den Ouweland A, Meijer-van Gelder ME, Portengen H, Klijn JG, Meijers-Heijboer H, Foekens JA, Schutte M (2009) CHEK2 1100delC and male breast cancer in the Netherlands. Breast Cancer Res Treat 116(2):397–400. https://doi.org/10.1007/s10549-008-0162-7
Antoniou AC, Casadei S, Heikkinen T, Barrowdale D, Pylkas K, Roberts J, Lee A, Subramanian D, De Leeneer K, Fostira F, Tomiak E, Neuhausen SL, Teo ZL, Khan S, Aittomaki K, Moilanen JS, Turnbull C, Seal S, Mannermaa A, Kallioniemi A, Lindeman GJ, Buys SS, Andrulis IL, Radice P, Tondini C, Manoukian S, Toland AE, Miron P, Weitzel JN, Domchek SM, Poppe B, Claes KB, Yannoukakos D, Concannon P, Bernstein JL, James PA, Easton DF, Goldgar DE, Hopper JL, Rahman N, Peterlongo P, Nevanlinna H, King MC, Couch FJ, Southey MC, Winqvist R, Foulkes WD, Tischkowitz M (2014) Breast-cancer risk in families with mutations in PALB2. N Engl J Med 371(6):497–506. https://doi.org/10.1056/NEJMoa1400382
Fackenthal JD, Marsh DJ, Richardson AL, Cummings SA, Eng C, Robinson BG, Olopade OI (2001) Male breast cancer in Cowden syndrome patients with germline PTEN mutations. J Med Genet 38(3):159–164
Lakshmaiah KC, Kumar AN, Purohit S, Viveka BK, Rajan KR, Zameer MAL, Namitha P, Saini ML, Azim HA Jr, Saini KS (2014) Neurofibromatosis type I with breast cancer: not only for women! Hered Cancer Clin Pract 12(1):5. https://doi.org/10.1186/1897-4287-12-5
Kurian AW, Hare EE, Mills MA, Kingham KE, McPherson L, Whittemore AS, McGuire V, Ladabaum U, Kobayashi Y, Lincoln SE, Cargill M, Ford JM (2014) Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol 32(19):2001–2009. https://doi.org/10.1200/JCO.2013.53.6607
Tung N, Battelli C, Allen B, Kaldate R, Bhatnagar S, Bowles K, Timms K, Garber JE, Herold C, Ellisen L, Krejdovsky J, DeLeonardis K, Sedgwick K, Soltis K, Roa B, Wenstrup RJ, Hartman AR (2015) Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer 121(1):25–33. https://doi.org/10.1002/cncr.29010
Kapoor NS, Curcio LD, Blakemore CA, Bremner AK, McFarland RE, West JG, Banks KC (2015) Multigene panel testing detects equal rates of pathogenic BRCA1/2 mutations and has a higher diagnostic yield compared to limited BRCA1/2 analysis alone in patients at risk for hereditary breast cancer. Ann Surg Oncol 22(10):3282–3288. https://doi.org/10.1245/s10434-015-4754-2
Desmond A, Kurian AW, Gabree M, Mills MA, Anderson MJ, Kobayashi Y, Horick N, Yang S, Shannon KM, Tung N, Ford JM, Lincoln SE, Ellisen LW (2015) Clinical actionability of multigene panel testing for hereditary breast and ovarian cancer risk assessment. JAMA Oncol 1(7):943–951. https://doi.org/10.1001/jamaoncol.2015.2690
Pritzlaff M, Summerour P, McFarland R, Li S, Reineke P, Dolinsky JS, Goldgar DE, Shimelis H, Couch FJ, Chao EC, LaDuca H (2017) Male breast cancer in a multi-gene panel testing cohort: insights and unexpected results. Breast Cancer Res Treat 161(3):575–586. https://doi.org/10.1007/s10549-016-4085-4
Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucl Acids Res 16(3):1215
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, Committee ALQA (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17(5):405–424. https://doi.org/10.1038/gim.2015.30
Silvestri V, Barrowdale D, Mulligan AM, Neuhausen SL, Fox S, Karlan BY, Mitchell G, James P, Thull DL, Zorn KK, Carter NJ, Nathanson KL, Domchek SM, Rebbeck TR, Ramus SJ, Nussbaum RL, Olopade OI, Rantala J, Yoon SY, Caligo MA, Spugnesi L, Bojesen A, Pedersen IS, Thomassen M, Jensen UB, Toland AE, Senter L, Andrulis IL, Glendon G, Hulick PJ, Imyanitov EN, Greene MH, Mai PL, Singer CF, Rappaport-Fuerhauser C, Kramer G, Vijai J, Offit K, Robson M, Lincoln A, Jacobs L, Machackova E, Foretova L, Navratilova M, Vasickova P, Couch FJ, Hallberg E, Ruddy KJ, Sharma P, Kim SW, kConFab I, Teixeira MR, Pinto P, Montagna M, Matricardi L, Arason A, Johannsson OT, Barkardottir RB, Jakubowska A, Lubinski J, Izquierdo A, Pujana MA, Balmana J, Diez O, Ivady G, Papp J, Olah E, Kwong A, Hereditary B, Ovarian Cancer Research Group N, Nevanlinna H, Aittomaki K, Perez Segura P, Caldes T, Van Maerken T, Poppe B, Claes KB, Isaacs C, Elan C, Lasset C, Stoppa-Lyonnet D, Barjhoux L, Belotti M, Meindl A, Gehrig A, Sutter C, Engel C, Niederacher D, Steinemann D, Hahnen E, Kast K, Arnold N, Varon-Mateeva R, Wand D, Godwin AK, Evans DG, Frost D, Perkins J, Adlard J, Izatt L, Platte R, Eeles R, Ellis S, Embrace Hamann U, Garber J, Fostira F, Fountzilas G, Pasini B, Giannini G, Rizzolo P, Russo A, Cortesi L, Papi L, Varesco L, Palli D, Zanna I, Savarese A, Radice P, Manoukian S, Peissel B, Barile M, Bonanni B, Viel A, Pensotti V, Tommasi S, Peterlongo P, Weitzel JN, Osorio A, Benitez J, McGuffog L, Healey S, Gerdes AM, Ejlertsen B, Hansen TV, Steele L, Ding YC, Tung N, Janavicius R, Goldgar DE, Buys SS, Daly MB, Bane A, Terry MB, John EM, Southey M, Easton DF, Chenevix-Trench G, Antoniou AC, Ottini L (2016) Male breast cancer in BRCA1 and BRCA2 mutation carriers: pathology data from the Consortium of Investigators of Modifiers of BRCA1/2. Breast Cancer Res 18(1):15. https://doi.org/10.1186/s13058-016-0671-y
Susswein LR, Marshall ML, Nusbaum R, Vogel Postula KJ, Weissman SM, Yackowski L, Vaccari EM, Bissonnette J, Booker JK, Cremona ML, Gibellini F, Murphy PD, Pineda-Alvarez DE, Pollevick GD, Xu Z, Richard G, Bale S, Klein RT, Hruska KS, Chung WK (2016) Pathogenic and likely pathogenic variant prevalence among the first 10,000 patients referred for next-generation cancer panel testing. Genet Med 18(8):823–832. https://doi.org/10.1038/gim.2015.166
Frank TS, Deffenbaugh AM, Reid JE, Hulick M, Ward BE, Lingenfelter B, Gumpper KL, Scholl T, Tavtigian SV, Pruss DR, Critchfield GC (2002) Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol 20(6):1480–1490. https://doi.org/10.1200/JCO.2002.20.6.1480
Ottini L, Silvestri V, Rizzolo P, Falchetti M, Zanna I, Saieva C, Masala G, Bianchi S, Manoukian S, Barile M, Peterlongo P, Varesco L, Tommasi S, Russo A, Giannini G, Cortesi L, Viel A, Montagna M, Radice P, Palli D (2012) Clinical and pathologic characteristics of BRCA-positive and BRCA-negative male breast cancer patients: results from a collaborative multicenter study in Italy. Breast Cancer Res Treat 134(1):411–418. https://doi.org/10.1007/s10549-012-2062-0
Konstantopoulou I, Tsitlaidou M, Fostira F, Pertesi M, Stavropoulou AV, Triantafyllidou O, Tsotra E, Tsiftsoglou AP, Tsionou C, Droufakou S, Dimitrakakis C, Fountzilas G, Yannoukakos D (2014) High prevalence of BRCA1 founder mutations in Greek breast/ovarian families. Clin Genet 85(1):36–42. https://doi.org/10.1111/cge.12274
Fostira F, Tsitlaidou M, Papadimitriou C, Pertesi M, Timotheadou E, Stavropoulou AV, Glentis S, Bournakis E, Bobos M, Pectasides D, Papakostas P, Pentheroudakis G, Gogas H, Skarlos P, Samantas E, Bafaloukos D, Kosmidis PA, Koutras A, Yannoukakos D, Konstantopoulou I, Fountzilas G (2012) Prevalence of BRCA1 mutations among 403 women with triple-negative breast cancer: implications for genetic screening selection criteria: a Hellenic Cooperative Oncology Group Study. Breast Cancer Res Treat 134(1):353–362. https://doi.org/10.1007/s10549-012-2021-9
Syrjakoski K, Kuukasjarvi T, Waltering K, Haraldsson K, Auvinen A, Borg A, Kainu T, Kallioniemi OP, Koivisto PA (2004) BRCA2 mutations in 154 finnish male breast cancer patients. Neoplasia 6(5):541–545. https://doi.org/10.1593/neo.04193
Renwick A, Thompson D, Seal S, Kelly P, Chagtai T, Ahmed M, North B, Jayatilake H, Barfoot R, Spanova K, McGuffog L, Evans DG, Eccles D, Breast Cancer Susceptibility C, Easton DF, Stratton MR, Rahman N (2006) ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat Genet 38(8):873–875. https://doi.org/10.1038/ng1837
Couch FJ, Shimelis H, Hu C, Hart SN, Polley EC, Na J, Hallberg E, Moore R, Thomas A, Lilyquist J, Feng B, McFarland R, Pesaran T, Huether R, LaDuca H, Chao EC, Goldgar DE, Dolinsky JS (2017) Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol 3(9):1190–1196. https://doi.org/10.1001/jamaoncol.2017.0424
Roberts NJ, Jiao Y, Yu J, Kopelovich L, Petersen GM, Bondy ML, Gallinger S, Schwartz AG, Syngal S, Cote ML, Axilbund J, Schulick R, Ali SZ, Eshleman JR, Velculescu VE, Goggins M, Vogelstein B, Papadopoulos N, Hruban RH, Kinzler KW, Klein AP (2012) ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov 2(1):41–46. https://doi.org/10.1158/2159-8290.CD-11-0194
Lilyquist J, LaDuca H, Polley E, Davis BT, Shimelis H, Hu C, Hart SN, Dolinsky JS, Couch FJ, Goldgar DE (2017) Frequency of mutations in a large series of clinically ascertained ovarian cancer cases tested on multi-gene panels compared to reference controls. Gynecol Oncol 147(2):375–380. https://doi.org/10.1016/j.ygyno.2017.08.030
Vahteristo P, Bartkova J, Eerola H, Syrjakoski K, Ojala S, Kilpivaara O, Tamminen A, Kononen J, Aittomaki K, Heikkila P, Holli K, Blomqvist C, Bartek J, Kallioniemi OP, Nevanlinna H (2002) A CHEK2 genetic variant contributing to a substantial fraction of familial breast cancer. Am J Hum Genet 71(2):432–438. https://doi.org/10.1086/341943
Hallamies S, Pelttari LM, Poikonen-Saksela P, Jekunen A, Jukkola-Vuorinen A, Auvinen P, Blomqvist C, Aittomaki K, Mattson J, Nevanlinna H (2017) CHEK2 c.1100delC mutation is associated with an increased risk for male breast cancer in Finnish patient population. BMC Cancer 17(1):620. https://doi.org/10.1186/s12885-017-3631-8
Apostolou P, Fostira F, Papamentzelopoulou M, Michelli M, Panopoulos C, Fountzilas G, Konstantopoulou I, Voutsinas GE, Yannoukakos D (2015) CHEK2 c.1100delC allele is rarely identified in Greek breast cancer cases. Cancer Genet 208(4):129–134. https://doi.org/10.1016/j.cancergen.2015.02.006
Zemankova P, Lhota F, Kleiblova P, Soukupova J, Vocka M, Janatova M, Kleibl Z (2016) RE: frameshift variant FANCL*c.1096_1099dupATTA is not associated with high breast cancer risk. Clin Genet 90(4):387–389. https://doi.org/10.1111/cge.12842
Pfeifer K, Schurmann P, Bogdanova N, Neuhauser K, Kostovska IM, Plaseska-Karanfilska D, Park-Simon TW, Schindler D, Dork T (2016) Frameshift variant FANCL*c.1096_1099dupATTA is not associated with high breast cancer risk. Clin Genet 90(4):385–386. https://doi.org/10.1111/cge.12837
Richards CS, Bale S, Bellissimo DB, Das S, Grody WW, Hegde MR, Lyon E, Ward BE, Molecular Subcommittee of the ALQAC (2008) ACMG recommendations for standards for interpretation and reporting of sequence variations: revisions 2007. Genet Med 10(4):294–300. https://doi.org/10.1097/gim.0b013e31816b5cae
ten Broeke SW, Brohet RM, Tops CM, van der Klift HM, Velthuizen ME, Bernstein I, Capella Munar G, Gomez Garcia E, Hoogerbrugge N, Letteboer TG, Menko FH, Lindblom A, Mensenkamp AR, Moller P, van Os TA, Rahner N, Redeker BJ, Sijmons RH, Spruijt L, Suerink M, Vos YJ, Wagner A, Hes FJ, Vasen HF, Nielsen M, Wijnen JT (2015) Lynch syndrome caused by germline PMS2 mutations: delineating the cancer risk. J Clin Oncol 33(4):319–325. https://doi.org/10.1200/JCO.2014.57.8088
Espenschied CR, LaDuca H, Li S, McFarland R, Gau CL, Hampel H (2017) Multigene panel testing provides a new perspective on lynch syndrome. J Clin Oncol 35(22):2568–2575. https://doi.org/10.1200/JCO.2016.71.9260
Bonadona V, Bonaiti B, Olschwang S, Grandjouan S, Huiart L, Longy M, Guimbaud R, Buecher B, Bignon YJ, Caron O, Colas C, Nogues C, Lejeune-Dumoulin S, Olivier-Faivre L, Polycarpe-Osaer F, Nguyen TD, Desseigne F, Saurin JC, Berthet P, Leroux D, Duffour J, Manouvrier S, Frebourg T, Sobol H, Lasset C, Bonaiti-Pellie C, French Cancer Genetics N (2011) Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA 305(22):2304–2310. https://doi.org/10.1001/jama.2011.743
Senter L, Clendenning M, Sotamaa K, Hampel H, Green J, Potter JD, Lindblom A, Lagerstedt K, Thibodeau SN, Lindor NM, Young J, Winship I, Dowty JG, White DM, Hopper JL, Baglietto L, Jenkins MA, de la Chapelle A (2008) The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology 135(2):419–428. https://doi.org/10.1053/j.gastro.2008.04.026
Ding YC, Steele L, Kuan CJ, Greilac S, Neuhausen SL (2011) Mutations in BRCA2 and PALB2 in male breast cancer cases from the United States. Breast Cancer Res Treat 126(3):771–778. https://doi.org/10.1007/s10549-010-1195-2
Acknowledgements
We are indebted to the patients who participated in our research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no disclosures/conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Fostira, F., Saloustros, E., Apostolou, P. et al. Germline deleterious mutations in genes other than BRCA2 are infrequent in male breast cancer. Breast Cancer Res Treat 169, 105–113 (2018). https://doi.org/10.1007/s10549-018-4661-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4661-x