Abstract
Purpose
Aromatase inhibitor-associated musculoskeletal symptoms (AIMSS) frequently occur in women being treated for breast cancer. Prior studies suggest high prevalence of vitamin D deficiency in breast cancer patients with musculoskeletal (MS) pain. We conducted a randomized, placebo-controlled trial to determine if 30,000 IU vitamin D3 per week (VitD3) would prevent worsening of AIMSS in women starting adjuvant letrozole for breast cancer.
Methods
Women with stage I–III breast cancer starting adjuvant letrozole and 25(OH)D level ≤40 ng/ml were eligible. All subjects received standard daily supplement of 1200 mg calcium and 600 IU vitamin D3 and were randomized to 30,000 IU oral VitD3/week or placebo. Pain, disability, fatigue, quality of life, 25(OH)D levels, and hand grip strength were assessed at baseline, 12, and 24 weeks. The primary endpoint was incidence of an AIMSS event.
Results
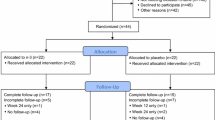
Median age of the 160 subjects (80/arm) was 61. Median 25OHD (ng/ml) was 25 at baseline, 32 at 12 weeks, and 31 at 24 weeks in the placebo arm and 22, 53, and 57 in the VitD3 arm. There were no serious adverse events. At week 24, 51% of women assigned to placebo had a protocol defined AIMSS event (worsening of joint pain using a categorical pain intensity scale (CPIS), disability from joint pain using HAQ-II, or discontinuation of letrozole due to MS symptoms) vs. 37% of women assigned to VitD3 (p = 0.069). When the brief pain inventory (BPI) was used instead of CPIS, the difference was statistically significant: 56 vs. 39% (p = 0.024).
Conclusions
Although 30,000 IU/week of oral vitamin D3 is safe and effective in achieving adequate vitamin D levels, it was not associated with a decrease in AIMSS events based on the primary endpoint. Post-hoc analysis using a different tool suggests potential benefit of vitamin D3 in reducing AIMSS.


Similar content being viewed by others
Abbreviations
- AIMSS:
-
Aromatase Inhibitor-Associated Musculoskeletal Symptoms
- AIST:
-
Aromatase Inhibitor Symptom Tool
- AIs:
-
Aromatase Inhibitors
- BFI:
-
Brief Fatigue Inventory
- BPI:
-
Brief Pain Inventory
- CPIS:
-
Categorical Pain Intensity Scale
- FACT-B:
-
Functional Assessment of Cancer Therapy-Breast
- HAQ-II:
-
Health Assessment Questionnaire II
- MENQOL:
-
Menopause-specific Quality of Life
- VITAL:
-
VITamin D treatment to prevent Arthralgia in women starting Letrozole
- VitD3:
-
30,000 IU vitamin D3 per week
References
Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, Sahmoud T, ATAC Trialists’ Group (2002) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 359:2131–2139
Coates AS, Keshaviah A, Thürlimann B, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Colleoni M, Láng I, Del Mastro L, Smith I, Chirgwin J, Nogaret JM, Pienkowski T, Wardley A, Jakobsen EH, Price KN, Goldhirsch A (2007) Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol 25:486–492
Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, Jones SE, Alvarez I, Bertelli G, Ortmann O, Coates AS, Bajetta E, Dodwell D, Coleman RE, Fallowfield LJ, Mickiewicz E, Andersen J, Lønning PE, Cocconi G, Stewart A, Stuart N, Snowdon CF, Carpentieri M, Massimini G, Bliss JM, van de Velde C, Intergroup Exemestane Study (2007) A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. New Engl J Med 350:1081–1092
Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D, Sierra A, Hershman DL (2007) Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol 25:3877–3883
Roberts K, Rickett K, Greer R, Woodward N (2017) Management of aromatase inhibitor induced musculoskeletal symptoms in postmenopausal early Breast cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol 111:66–80
Plotnikoff GA, Quigley JM (2003) Prevalence of severe hypovitaminosis D in patients with persistent nonspecific musculoskeletal pain. Mayo Clin Proc 78:1463–1470
Taylor M, Rastelli A, Civitelli R et al. (2004) Incidence of 25-OH vitamin D deficiency in patients with a history of breast cancer who have musculoskeletal symptomatology. 27th Annual San Antonio Breast Cancer Symposium,San Antonio, TX, 8–11 Dec 2004 (abstract 3072)
Crew KD, Shane E, Cremers S, McMahon DJ, Irani D, Hershman DL (2009) High prevalence of vitamin D deficiency despite supplementation in premenopausal women with breast cancer undergoing adjuvant chemotherapy. J Clin Oncol 27:2151–2156
de Torrenté de la Jara G, Pécoud A, Favrat B (2004) Musculoskeletal pain in female asylum seekers and hypovitaminosis D3. BMJ 329:156–157
Caniggia A, Lorè F, di Cairano G, Nuti R (1987) Main endocrine modulators of vitamin D hydroxylases in human pathophysiology. J Steroid Biochem 27:815–824
Gilad LA, Bresler T, Gnainsky J, Smirnoff P, Schwartz B (2005) Regulation of vitamin D receptor expression via estrogen-induced activation of the ERK 1/2 signaling pathway in colon and breast cancer cells. J Endocrinol 185:577–592
Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B (2005) Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 84:18–28
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357(3):266–281
Khan QJ, Reddy PS, Kimler BF, Sharma P, Baxa SE, O’Dea AP, Klemp JR, Fabian CJ (2010) Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res Treat 119:111–118
Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A (2004) Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) adjuvant breast cancer trial. J Clin Oncol 22:4261–4271
Whelan TJ, Goss PE, Ingle JN, Pater JL, Tu D, Pritchard K, Liu S, Shepherd LE, Palmer M, Robert NJ, Martino S, Muss HB (2005) Assessment of quality of life in MA.17: a randomized, placebo-controlled trial of letrozole after 5 years of tamoxifen in postmenopausal women. J Clin Oncol 23:6931–6940
Fallowfield LJ, Bliss JM, Porter LS, Price MH, Snowdon CF, Jones SE, Coombes RC, Hall E (2006) Quality of life in the intergroup exemestane study: a randomized trial of exemestane versus continued tamoxifen after 2 to 3 years of tamoxifen in postmenopausal women with primary breast cancer. J Clin Oncol 24:910–917
Cecil RL, Archer BH (1925) Arthritis of the menopause. A study of fifty cases. JAMA 84:75–79
Felson DT, Cummings SR (2005) Aromatase inhibitors and the syndrome of arthralgias with estrogen deprivation. Arthritis Rheum 52:2594–2598
Vegeto E, Bonincontro C, Pollio G, Sala A, Viappiani S, Nardi F, Brusadelli A, Viviani B, Ciana P, Maggi A (2001) Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J Neurosci 21:1809–1818
Felson DT, Niu J, Clancy M, Aliabadi P, Sack B, Guermazi A, Hunter DJ, Amin S, Rogers G, Booth S (2007) Low levels of vitamin D and worsening of knee osteoarthritis: results of two longitudinal studies. Arthritis Rheum 56:129–136
Morales L, Pans S, Verschueren K, Van Calster B, Paridaens R, Westhovens R, Timmerman D, De Smet L, Vergote I, Christiaens MR, Neven P (2008) Prospective study to assess short-term intra-articular and tenosynovial changes in the aromatase inhibitor–associated arthralgia syndrome. J Clin Oncol 26:3147–3152
Hayes CE, Nashold FE, Spach KM, Pedersen LB (2003) The immunological functions of the vitamin D endocrine system. Cell Mol Biol 49:277–300
Cheema C, Grant BF, Marcus R (1989) Effects of estrogen on circulating “free” and total 1,25-dihydroxyvitamin D and on the parathyroid-vitamin D axis in postmenopausal women. J Clin Invest 83:537–542
Buchanan JR, Santen R, Cauffman S, Cavaliere A, Greer RB, Demers LM (1986) The effect of endogenous estrogen fluctuation on metabolism of 25-hydroxyvitamin D. Calcif Tissue Int 39:139–144
Rastelli AL, Taylor ME, Gao F, Armamento-Villareal R, Jamalabadi-Majidi S, Napoli N, Ellis MJ (2011) Vitamin D and aromatase inhibitor-induced musculoskeletal symptoms (AIMSS): a phase II, double-blind, placebo-controlled, randomized trial. Breast Cancer Res Treat 129:107–116
Acknowledgements
Letrozole and funding for this study were provided by Novartis Pharmaceutical Corporation (East Hanover, NJ). Vitamin D3 (10,000 IU capsules) and matched, blinded placebo were provided by BTR Group, Inc. (Pittsfield, IL). Neither company was the sponsor for the trial; nor were they involved in any aspect of design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The following authors are without financial interests or conflicts of interest related to this trial: Kimler, Reddy, Klemp, Nydegger, and Yeh. Within the past three years, Dr. Khan has served as a consultant to Novartis Pharmaceutical Company, Inc. and Pfizer. Drs. Khan and Sharma have received during the past three years, via their institution, funding for support of research and clinical trials from the following companies: AstraZeneca; Bristol-Myers Squibb; Celgene, Inc.; Novartis Pharmaceutical Company, Inc.; Genentech-Roche, GlaxoSmithKline, and Pfizer. Study agent but no funding has been provided by DSM and Pfizer for trials conducted by Dr. Fabian.
Ethical standards
The conduct of the trial complies with the current laws of the United States of America.
Rights and permissions
About this article
Cite this article
Khan, Q.J., Kimler, B.F., Reddy, P.S. et al. Randomized trial of vitamin D3 to prevent worsening of musculoskeletal symptoms in women with breast cancer receiving adjuvant letrozole. The VITAL trial. Breast Cancer Res Treat 166, 491–500 (2017). https://doi.org/10.1007/s10549-017-4429-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4429-8