Abstract
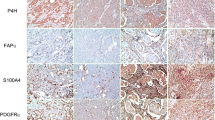
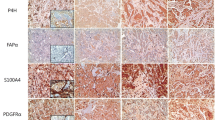
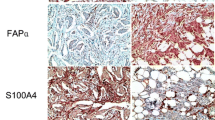
Cancer-associated fibroblasts (CAFs) are classified into various functional subtypes such as fibroblast activation protein-α (FAP-α), fibroblast specific protein-1 (FSP-1), platelet-derived growth factor receptor-α (PDGFR-α), and PDGFR-β. In this study, we compared the expression of CAF-related proteins in invasive lobular carcinoma (ILC) with those in invasive carcinoma of no special type (NST) and assessed the implications of the differences observed. Using tissue microarrays of 104 ILC and 524 invasive carcinoma (NST) cases, immunohistochemistry for CAF-related proteins [podoplanin, prolyl 4-hydroxylase, FAP-α, FSP-1/S100A4, PDGFR-α, PDGFR-β, and chondroitin sulfate proteoglycan (NG2)] was conducted. In invasive carcinoma (NST), tumor cells expressed a high level of PDGFR-α, whereas ILC tumor cells expressed high levels of podoplanin, prolyl 4-hydroxylase, FAP-α, and FSP-1/S100A4. In stromal cells of invasive carcinoma (NST), high expression levels of prolyl 4-hydroxylase, PDGFR-α, and NG2 were observed, whereas ILC stromal cells expressed high levels of FAP-α, FSP-1/S100A4, and PDGFR-β. In ILC, tumoral FSP-1/S100A4 positivity was associated with higher Ki-67 labeling index (p = 0.010) and non-luminal A type cancer (p = 0.014). Stromal PDGFR-α positivity was associated with lymph node metastasis (p = 0.011). On survival analysis of entire cases, tumoral FSP-1/S100A4 positivity (p = 0.002), stromal podoplanin positivity (p = 0.041), and stromal FSP-1/S100A4 negativity (p = 0.041) were associated with shorter disease-free survival; only tumoral FSP-1/S100A4 positivity (p = 0.044) was associated with shorter overall survival. In ILC, the expression of FAP-α and FSP-1/S100A4 was higher in both tumor and stromal cells than that observed in invasive carcinoma (NST). These results indicate that CAFs are a potential target in ILC treatment.




Similar content being viewed by others
References
Tavassoli FA, Devilee P, International Agency for Research on Cancer et al (2003) Pathology and genetics of tumours of the breast and female genital organs. IAPS Press, Lyon
Li CI, Anderson BO, Daling JR et al (2003) Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA 289(11):1421–1424
Li CI, Uribe DJ, Daling JR (2005) Clinical characteristics of different histologic types of breast cancer. Br J Cancer 93(9):1046–1052. doi:10.1038/sj.bjc.6602787
Tot T (2000) The cytokeratin profile of medullary carcinoma of the breast. Histopathology 37(2):175–181. doi:10.1046/j.1365-2559.2000.00889.x
Reeves GK, Beral V, Green J et al (2006) Hormonal therapy for menopause and breast-cancer risk by histological type: a cohort study and meta-analysis. Lancet Oncol 7(11):910–918. doi:10.1016/s1470-2045(06)70911-1
Lesser ML, Rosen PP, Kinne DW (1982) Multicentricity and bilaterality in invasive breast carcinoma. Surgery 91(2):234–240
Silverstein MJ, Lewinsky BS, Waisman JR et al (1994) Infiltrating lobular carcinoma. Is it different from infiltrating duct carcinoma? Cancer 73(6):1673–1677
De Leeuw WJ, Berx G, Vos CB et al (1997) Simultaneous loss of E-cadherin and catenins in invasive lobular breast cancer and lobular carcinoma in situ. J Pathol 183(4):404–411. doi:10.1002/(sici)1096-9896(199712)183:4<404:aid-path1148>3.0.co;2-9
Tsutsui S, Ohno S, Murakami S et al (2003) Prognostic value of the combination of epidermal growth factor receptor and c-erbB-2 in breast cancer. Surgery 133(2):219–221. doi:10.1067/msy.2003.32
Uehara H, Takahashi T, Oha M et al (2014) Exogenous fatty acid binding protein 4 promotes human prostate cancer cell progression. Int J Cancer. doi:10.1002/ijc.28903
Franco OE, Shaw AK, Strand DW et al (2010) Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol 21(1):33–39. doi:10.1016/j.semcdb.2009.10.010
Ostman A (2014) Cancer-associated fibroblasts: recent developments and emerging challenges. Semin Cancer Biol 25:1–2. doi:10.1016/j.semcancer.2014.02.004
Desmouliere A, Guyot C, Gabbiani G (2004) The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol 48(5–6):509–517. doi:10.1387/ijdb.041802ad
De Wever O, Nguyen QD, Van Hoorde L et al (2004) Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. FASEB J 18(9):1016–1018. doi:10.1096/fj.03-1110fje
Sugimoto H, Mundel TM, Kieran MW et al (2006) Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther 5(12):1640–1646
Pietras K, Sjoblom T, Rubin K et al (2003) PDGF receptors as cancer drug targets. Cancer Cell 3(5):439–443
Kraman M, Bambrough PJ, Arnold JN et al (2010) Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 330(6005):827–830. doi:10.1126/science.1195300
Kawase A, Ishii G, Nagai K et al (2008) Podoplanin expression by cancer associated fibroblasts predicts poor prognosis of lung adenocarcinoma. Int J Cancer 123(5):1053–1059. doi:10.1002/ijc.23611
Kojima Y, Acar A, Eaton EN et al (2010) Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci USA 107(46):20009–20014. doi:10.1073/pnas.1013805107
Cortez E, Roswall P, Pietras K (2014) Functional subsets of mesenchymal cell types in the tumor microenvironment. Semin Cancer Biol 25:3–9. doi:10.1016/j.semcancer.2013.12.010
Mao Y, Keller ET, Garfield DH et al (2013) Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev 32(1–2):303–315. doi:10.1007/s10555-012-9415-3
Nakagawa S, Miki Y, Miyashita M et al (2016) Tumor microenvironment in invasive lobular carcinoma: possible therapeutic targets. Breast Cancer Res Treat 155(1):65–75. doi:10.1007/s10549-015-3668-9
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19(5):403–410
Hammond ME, Hayes DF, Dowsett M et al (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28(16):2784–2795. doi:10.1200/jco.2009.25.6529
Wolff AC, Hammond ME, Hicks DG et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31(31):3997–4013. doi:10.1200/jco.2013.50.9984
Henry LR, Lee HO, Lee JS et al (2007) Clinical implications of fibroblast activation protein in patients with colon cancer. Clin Cancer Res 13(6):1736–1741. doi:10.1158/1078-0432.ccr-06-1746
Goldhirsch A, Wood WC, Coates AS et al (2011) Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 22(8):1736–1747. doi:10.1093/annonc/mdr304
Jansson S, Bendahl PO, Grabau DA et al (2014) The three receptor tyrosine kinases c-KIT, VEGFR2 and PDGFRalpha, closely spaced at 4q12, show increased protein expression in triple-negative breast cancer. Plos One 9(7):e102176. doi:10.1371/journal.pone.0102176
Zhu Y, Wang Y, Guan B et al (2014) C-kit and PDGFRA gene mutations in triple negative breast cancer. Int J Clin Exp Pathol 7(7):4280–4285
Martin-Villar E, Megias D, Castel S et al (2006) Podoplanin binds ERM proteins to activate RhoA and promote epithelial-mesenchymal transition. J Cell Sci 119(Pt 21):4541–4553. doi:10.1242/jcs.03218
Grau SJ, Trillsch F, Tonn JC et al (2015) Podoplanin increases migration and angiogenesis in malignant glioma. Int J Clin Exp Pathol 8(7):8663–8670
Xiong G, Deng L, Zhu J et al (2014) Prolyl-4-hydroxylase alpha subunit 2 promotes breast cancer progression and metastasis by regulating collagen deposition. BMC Cancer 14:1. doi:10.1186/1471-2407-14-1
Wasif N, Maggard MA, Ko CY et al (2010) Invasive lobular vs. ductal breast cancer: a stage-matched comparison of outcomes. Ann Surg Oncol 17(7):1862–1869. doi:10.1245/s10434-010-0953-z
Ito M, Hagiyama M, Mimae T et al (2014) alpha-Parvin, a pseudopodial constituent, promotes cell motility and is associated with lymph node metastasis of lobular breast carcinoma. Breast Cancer Res Treat 144(1):59–69. doi:10.1007/s10549-014-2859-0
Catteau X, Simon P, Noel JC (2014) Myofibroblastic stromal reaction and lymph node status in invasive breast carcinoma: possible role of the TGF-beta1/TGF-betaR1 pathway. BMC Cancer 14:499. doi:10.1186/1471-2407-14-499
Song S, Ewald AJ, Stallcup W et al (2005) PDGFRbeta + perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol 7(9):870–879. doi:10.1038/ncb1288
Iacobuzio-Donahue CA, Argani P, Hempen PM et al (2002) The desmoplastic response to infiltrating breast carcinoma: gene expression at the site of primary invasion and implications for comparisons between tumor types. Cancer Res 62(18):5351–5357
Shao ZM, Nguyen M, Barsky SH (2000) Human breast carcinoma desmoplasia is PDGF initiated. Oncogene 19(38):4337–4345. doi:10.1038/sj.onc.1203785
Huang Y, Simms AE, Mazur A et al (2011) Fibroblast activation protein-alpha promotes tumor growth and invasion of breast cancer cells through non-enzymatic functions. Clin Exp Metastasis 28(6):567–579. doi:10.1007/s10585-011-9392-x
Jia J, Martin TA, Ye L et al (2014) FAP-alpha (Fibroblast activation protein-alpha) is involved in the control of human breast cancer cell line growth and motility via the FAK pathway. BMC Cell Biol 15:16. doi:10.1186/1471-2121-15-16
Jenkinson SR, Barraclough R, West CR et al (2004) S100A4 regulates cell motility and invasion in an in vitro model for breast cancer metastasis. Br J Cancer 90(1):253–262. doi:10.1038/sj.bjc.6601483
Wang L, Wang X, Liang Y et al (2012) S100A4 promotes invasion and angiogenesis in breast cancer MDA-MB-231 cells by upregulating matrix metalloproteinase-13. Acta Biochim Pol 59(4):593–598
Michaut M, Chin SF, Majewski I et al (2016) Integration of genomic, transcriptomic and proteomic data identifies two biologically distinct subtypes of invasive lobular breast cancer. Sci Rep 6:18517. doi:10.1038/srep18517
O’Connell JT, Sugimoto H, Cooke VG et al (2011) VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc Natl Acad Sci USA 108(38):16002–16007. doi:10.1073/pnas.1109493108
Zhang J, Chen L, Liu X et al (2013) Fibroblast-specific protein 1/S100A4-positive cells prevent carcinoma through collagen production and encapsulation of carcinogens. Cancer Res 73(9):2770–2781. doi:10.1158/0008-5472.can-12-3022
Cabezon T, Celis JE, Skibshoj I et al (2007) Expression of S100A4 by a variety of cell types present in the tumor microenvironment of human breast cancer. Int J Cancer 121(7):1433–1444. doi:10.1002/ijc.22850
Zhang J, Chen L, Xiao M et al (2011) FSP1 + fibroblasts promote skin carcinogenesis by maintaining MCP-1-mediated macrophage infiltration and chronic inflammation. Am J Pathol 178(1):382–390. doi:10.1016/j.ajpath.2010.11.017
Eusebi V, Magalhaes F, Azzopardi JG (1992) Pleomorphic lobular carcinoma of the breast: an aggressive tumor showing apocrine differentiation. Hum Pathol 23(6):655–662
Bentz JS, Yassa N, Clayton F (1998) Pleomorphic lobular carcinoma of the breast: clinicopathologic features of 12 cases. Mod Pathol 11(9):814–822
Narendra S, Jenkins SM, Khoor A et al (2015) Clinical outcome in pleomorphic lobular carcinoma: a case-control study with comparison to classic invasive lobular carcinoma. Ann Diagn Pathol 19(2):64–69. doi:10.1016/j.anndiagpath.2015.01.005
Simpson PT, Reis-Filho JS, Lambros MB et al (2008) Molecular profiling pleomorphic lobular carcinomas of the breast: evidence for a common molecular genetic pathway with classic lobular carcinomas. J Pathol 215(3):231–244. doi:10.1002/path.2358
Ohlund D, Elyada E, Tuveson D (2014) Fibroblast heterogeneity in the cancer wound. J Exp Med 211(8):1503–1523. doi:10.1084/jem.20140692
Haubeiss S, Schmid JO, Murdter TE et al (2010) Dasatinib reverses cancer-associated fibroblasts (CAFs) from primary lung carcinomas to a phenotype comparable to that of normal fibroblasts. Mol Cancer 9:168. doi:10.1186/1476-4598-9-168
Brennen WN, Isaacs JT, Denmeade SR (2012) Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol Cancer Ther 11(2):257–266. doi:10.1158/1535-7163.mct-11-0340
Santos AM, Jung J, Aziz N et al (2009) Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest 119(12):3613–3625. doi:10.1172/jci38988
Scott AM, Wiseman G, Welt S et al (2003) A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin Cancer Res 9(5):1639–1647
Neri S, Ishii G, Hashimoto H et al (2015) Podoplanin-expressing cancer-associated fibroblasts lead and enhance the local invasion of cancer cells in lung adenocarcinoma. Int J Cancer 137(4):784–796. doi:10.1002/ijc.29464
Slany A, Haudek-Prinz V, Meshcheryakova A et al (2014) Extracellular matrix remodeling by bone marrow fibroblast-like cells correlates with disease progression in multiple myeloma. J Proteome Res 13(2):844–854. doi:10.1021/pr400881p
Garin-Chesa P, Old LJ, Rettig WJ (1990) Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci USA 87(18):7235–7239
Lee HO, Mullins SR, Franco-Barraza J et al (2011) FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC Cancer 11:245. doi:10.1186/1471-2407-11-245
Liao D, Luo Y, Markowitz D et al (2009) Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. Plos One 4(11):e7965. doi:10.1371/journal.pone.0007965
Erez N, Truitt M, Olson P et al (2010) Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell 17(2):135–147. doi:10.1016/j.ccr.2009.12.041
Pietras K, Pahler J, Bergers G et al (2008) Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. Plos Med 5(1):e19. doi:10.1371/journal.pmed.0050019
Crawford Y, Kasman I, Yu L et al (2009) PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell 15(1):21–34. doi:10.1016/j.ccr.2008.12.004
Ehnman M, Missiaglia E, Folestad E et al (2013) Distinct effects of ligand-induced PDGFRalpha and PDGFRbeta signaling in the human rhabdomyosarcoma tumor cell and stroma cell compartments. Cancer Res 73(7):2139–2149. doi:10.1158/0008-5472.can-12-1646
Pietras K, Ostman A, Sjoquist M et al (2001) Inhibition of platelet-derived growth factor receptors reduces interstitial hypertension and increases transcapillary transport in tumors. Cancer Res 61(7):2929–2934
Pietras K, Rubin K, Sjoblom T et al (2002) Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res 62(19):5476–5484
Cooke VG, LeBleu VS, Keskin D et al (2012) Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell 21(1):66–81. doi:10.1016/j.ccr.2011.11.024
Funding
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1420080). This research was also supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning (2015R1A1A1A05001209).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Park, C.K., Jung, W.H. & Koo, J.S. Expression of cancer-associated fibroblast-related proteins differs between invasive lobular carcinoma and invasive ductal carcinoma. Breast Cancer Res Treat 159, 55–69 (2016). https://doi.org/10.1007/s10549-016-3929-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3929-2