Abstract
The TACT trial is the largest study assessing the benefit of taxanes as part of adjuvant therapy for early breast cancer. The goal of this translational study was to clarify the predictive and prognostic value of Tau within the TACT trial. Tissue microarrays (TMA) were available from 3,610 patients. ER, PR, HER2 from the TACT trial and Tau protein expression was determined by immunohistochemistry on duplicate TMAs. Two parallel scoring systems were generated for Tau expression (‘dichotomised’ vs. ‘combined’ score). The positivity rate of Tau expression was 50 % in the trial population (n = 2,483). Tau expression correlated positively with ER (p < 0.001) and PR status (p < 0.001); but negatively with histological grade (p < 0.001) and HER2 status (p < 0.001). Analyses with either scoring systems for Tau expression demonstrated no significant interaction between Tau expression and efficacy of docetaxel. Contrary to the hypothesis that taxane benefit would be enriched in Tau negative/low patients, the only groups with a suggestion of a reduced event rate in the taxane group were the HER2-positive, Tau positive subgroups. Tau expression was seen to be a prognostic factor on univariate analysis associated with an improved DFS, independent of the treatment group (p < 0.001). It had no prognostic value in ER-negative tumours and the weak prognostic effect of Tau in ER-positive tumours (p = 0.02) diminished, when considering ER as an ordinal variable. On multivariable analyses, Tau had no prognostic value in either group. In addition, no significant interaction between Tau expression and benefit from docetaxel in patients within the PR-positive and negative subsets was seen. This is now the second large adjuvant study, and the first with quantitative analysis of ER and Tau expression, failing to show an association between Tau and taxane benefit with limited utility as a prognostic marker for Tau in ER-positive early breast cancer patients.
Similar content being viewed by others
Introduction
Taxanes are potent cytotoxic compounds that have become a standard component of many adjuvant and neoadjuvant chemotherapy regimens for early breast cancer [1–5]. Overview analyses have suggested a small but clear incremental benefit for taxane-based therapy [6]. However, the addition of taxanes to cytotoxic regimens has not always demonstrated a consistent improvement in outcomes. Some studies have clearly shown an overall survival (OS) benefit, others an improvement in disease-free survival (DFS) but not OS, while others have shown no benefit [6]. Explanations for the variable evidence of additional therapeutic efficacy may include differences in sequence, duration and choice of control regimen. The UK Taxotere as Adjuvant Chemotherapy Trial (TACT) assessed the benefit of taxanes as part of an adjuvant therapy for early breast cancer patients and did not demonstrate an improvement in DFS, its primary endpoint measure [7, 8]. In the TACT trial treatment duration was similar in the comparator groups. The EBCTCG meta-analyses of adjuvant taxane treatment (n = 44,000 in 33 studies) confirmed that in trials where more anthracycline was given in the control groups to balance the treatment duration of taxanes, no significant difference in breast cancer mortality in favour of taxanes was observed, but in trials in which four cycles of a taxane were added to a fixed anthracycline-based regimen, breast cancer mortality decreased [9].
In addition to differences in clinical trial design and demographic differences in trial patient populations, the biological heterogeneity between patients’ tumours in relation to sensitivity and resistance to microtubule inhibition is likely to be critical in determining taxane benefit. Identification of definitive biomarkers to predict which patients benefit from taxane therapy and, conversely, which can be spared the cytotoxic effects of such treatments would be an important improvement in patient care [10]. Taxanes are known to exert their cytotoxic activity by interfering with spindle microtubule dynamics causing G2-M interphase cell cycle arrest, inducing subsequent apoptosis of tumour cells [3, 11]. Microtubule-associated proteins (MAPs) are endogenous proteins which participate in the organization, stabilization and function of the microtubules [12], and have, therefore, been under investigation as candidate markers to predict response to taxane therapy.
MAP-Tau protein (50–64 KDa) binds to the same pocket as taxanes in microtubules, thus competing for the drug-binding site [13–15]. In vitro experiments with small interfering RNAs have indicated that suppression of Tau increases the sensitivity of breast cancer cells to taxanes [16, 17], however, results from clinical studies evaluating Tau as a predictive biomarker for taxane sensitivity have been conflicting. Early studies of neoadjuvant taxane therapy reported a significant correlation between low Tau protein expression and higher pathological complete response (pCR) rate [16, 18]. Although this association has been supported by other clinical studies in the metastatic setting [19, 20]; no correlation between Tau gene/protein expression levels and efficacy of taxanes was observed in the retrospective subset analyses of the neoadjuvant GeparTrio [21], the adjuvant Hellenic Cooperative Oncology Group (HECOG) [22] or the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-28 trials [23].
Tau mRNA expression is induced by oestrogen, as well as tamoxifen, and correlates with oestrogen receptor (ER) expression [18, 22, 24]. It is possible that greater paclitaxel treatment effects observed in patients with Tau negative, ER-positive tumours who also received hormonal therapy may be skewed by the greater effect of addition of a taxane in those with less endocrine therapy sensitive disease. High Tau mRNA expression was shown to be significantly associated with reduced risk of recurrence (at both 5 and 10 years, p = 0.005 and p = 0.05, respectively) in patients treated with tamoxifen, indicating a potential predictive value of high Tau expression for endocrine therapy [18]. However, the positive association between Tau and ER expression within ER-positive tumours may explain this observation, addressing this question was one of the motivations of the current study.
As well as linking high Tau expression with endocrine sensitive disease and poorer response to chemotherapy, high Tau expression has been shown to be an independent good prognostic factor in some series [18, 20, 22, 23, 25]. In NSABP B-28 study (n = 1,942), a positive prognostic effect of high Tau expression was found in patients with ER-positive tumours. However, there were too few (n = 97) Tau positive patients in the ER-negative group to confidently address this question for this subgroup [23]. In contrast, a more recent study by Baquero et al. [26], reporting on the Yale University breast cancer cohort (n = 651) consisting of 57 % ER-negative cases, Tau showed a prognostic value in patients with ER-negative tumours. It is noteworthy that the immunohistochemistry (IHC) staining and scoring procedures were fundamentally different between the two studies. The relationship between ER and Tau in relation to prognosis and taxane treatment benefit prediction thus remains unclear and further study with separate analyses of ER-positive and negative tumours, and quantitative analyses of degree of expression of these biomarkers, are required to explore the Tau and oestrogen association in more detail. The TACT trial translational tissue database provides an appropriate test-bed for assessing biomarkers such as Tau. The goal of this translational study was to clarify the prognostic and predictive value of Tau using tissue specimens from patients enrolled into TACT.
Patients and methods
This study is reported in accordance with REMARK criteria [27], and biomarker analysis was performed in a GCLP-compliant facility.
Patients
A total of 4,162 patients with node-positive or high-risk node-negative operable early breast cancer were randomised within the TACT trial to FEC (fluorouracil 600 mg/m2, epirubicin 60 mg/m2 and cyclophosphamide 600 mg/m2) four cycles followed by docetaxel (100 mg/m2) four cycles, or control. Control regimens were FEC60 for a total of eight cycles or epirubicin (100 mg/m2) for four cycles followed by classical CMF (cyclophosphamide 600 mg/m2, methotrexate 40 mg/m2 and fluorouracil 600 mg/m2) for four cycles [7].
TMA construction and immunohistochemistry
A representative formalin-fixed, paraffin-embedded (FFPE) block of invasive breast tumour was requested for each patient who consented to its collection for research in a prospectively planned programme for translational biomarker evaluation within the TACT trial cohort ‘transTACT’, as described in [28]. From each FFPE block, four 0.6 mm cores of invasive tumour were selected to create a tissue microarray (TMA). Within the TACT patient population, tumour tissues from 3,610 patients (87 % of total) were available for TMA construction. Each TMA block contained between 100 and 200 cores of tissue, depending upon the array design. Clinical and pathological data [age, histological grade, invasive tumour size, lymph node status, ER and progesterone receptor (PR) status, disease outcome data] were collected within the TACT trial. Central HER2 testing was carried out, as described elsewhere [29]. Sections from duplicate TMA blocks composed of 0.6 mm tumour cores were used to assess Tau protein expression using IHC. The IHC procedure was centralized with sections processed within 5 days.
TMA sections were dewaxed and rehydrated. Antigen retrieval was achieved using the Pascal pressurized retrieval unit with Dako Target Retrieval Solution (pH 6). Sections were transferred to an automated staining system (BioGenex i6000) and the following antibodies applied; Tau (US Biological T1029, 1:50), ER (Labvision SP-1, 1:150), PR (Dako PgR636, 1:400). Following incubation, bound antibody was detected using the Envision/HRP kit (Dako, Denmark) and sections counterstained with haematoxylin.
Assessment of ER, PR and Tau expression
Staining for ER, PR and Tau was performed at Guy’s and St Thomas’ Hospital on duplicate TMAs and central specialist review was separately undertaken by two of CG, SEP, JB and AH for each marker, without reference to treatment allocation or clinical outcome. ER (by CG and SEP) and PR (by JB and AH) were quantified using the Allred score [30], resulting in a scale of 0–8. For ER and PR, a score of 3 or more was considered positive [30].
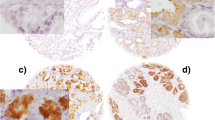
For Tau expression (assessed by CG and SEP) both the intensity (negative, weak, moderate or strong) and proportion of invasive tumour cells showing cytoplasmic staining (in 5 % increments) were recorded (Fig. 1). Cores with less than 20 invasive cells were excluded as insufficient for assessment. If there was disagreement between the two observers on intensity score, or if proportion differed by more than 10 %, the cases were jointly re-assessed by CG and SEP and consensus reached. For each marker, the rounded average of the scores from the duplicate TMAs was regarded as the final result. An ‘average Allred’ score of 1, representing the average of duplicate cores with Allred scores of 0 and 2 was, therefore, obtained for a small number of cases.
Representative immunohistochemical staining of Tau expression in tissue microarrays constructed from the TACT trial patient population. A Low-power tissue microarray showing variable expression between tumour cores. B Strong expression (strong, 100 %). C Moderate expression (Moderate, 95 %). D Weak expression (Weak, 80 %). E No expression (Negative). F Intra-tumour variability demonstrating strong and no expression. G Intra-tumour variability showing weak and moderate expression
Two parallel scoring systems were generated for the Tau expression: a) a ‘dichotomised score’ of negative versus positive, where Tau positive cases were defined as those demonstrating any expression and b) a ‘combined Allred score’ summing the intensity and the proportion of cytoplasmic staining, where intensity was scored 0–3, and proportion staining converted into a 0–5 score (0 % = 0, <1 % = 1, 1–10 % = 2, 11–33 % = 3, 34–65 % = 4 and 66–100 % = 5), thus representing Tau as a semi-quantitative categorical variable with scores from 0 to 8. For simplicity, when illustrating the distribution of Tau by other characteristics and displaying the results of multivariable analysis, the Tau combined score was classified into three groups: ‘negative’ with a score of 0; ‘intermediate’ with a score of 1–6 and ‘high’ with a score of 7–8.
Statistical analysis
DFS was the primary endpoint of the study, defined as time from randomisation to first invasive relapse, new primary invasive breast cancer (ipsilateral or contralateral), or death from any cause; patients who remained alive and disease free at their date of last follow up being censored at that point. Kaplan–Meier curves were plotted for survival endpoints, and treatment groups compared by use of the log-rank test. Hazard ratios (HRs) (with 95 % CIs) were obtained from Cox proportional hazards regression models with a HR less than 1 favouring the experimental regimen (FEC-T). The proportionality assumption of the Cox model was tested by fitting time-dependent effects (proportional to log (follow up time) for each factor using the TVC command in STATA. Unless stated otherwise, all analyses were unadjusted and stratified by centre’s choice of control regimen. All patients as randomised on an intention-to-treat basis were included in a specific analysis, if they had the factors required for that analysis successfully measured. A significance level of p < 0.05 was considered significant. This analysis is based on a database snapshot frozen on 25th November 2011 [8]. All analyses were done in STATA 11.2 for windows. The TACT trial is registered as an International Standard Randomised Controlled Trial, number ISRCTN79718493/CRUK01/001. With 796 events an interaction equivalent to a HR (taxane group: non-taxane group) of 0.76 in patients with Tau negative tumours and 1.08 in patients with Tau positive tumours would be detectable with 80 % power, one-sided 5 % significance level, these levels have been chosen to be compatible with the overall HR of 0.92.
Results
Patient characteristics and Tau expression
Sixty-nine percent (n = 2,483) of the tumours from the total of 3,610 cases in the TMAs were assessable for ER, PR, HER2 and Tau. Biomarker assessable patients showed very similar patient characteristics to the overall trial population (Supplementary Table 1). The median follow up time was 8.2 years. A very strong association was observed between the intensity and the proportion of Tau staining (p < 0.001) (Fig. 2). Tau positive status, defined as demonstrating at least weak intensity staining was assigned to 50 % of cases in the dichotomized scoring analysis (n = 1,242). The combined scoring system yielded the three Tau groups as described: negative—45 % 281 (n = 1,124) of the whole population, intermediate—26 % 282 (n = 647), and high—29 % (n = 712). Tau positive status correlated positively with ER status (p < 0.001) and PR status (p < 0.001) (Table 1; Fig. 3A, B, respectively). In contrast, Tau positive status correlated negatively with histological grade (p < 0.001) (from the local histopathology reports) and central HER2 status (p < 0.001). Tau expression did not show a significant correlation with age or tumour size but was weakly correlated with nodal status (Table 1).
Relationship between intensity and proportion of Tau staining. The number of cases is depicted along the vertical y axis. Subgroups of intensity of staining are depicted along the horizontal x axis. The percentage of cell staining within tissue sections is depicted along the z axis. Tau positive status in the dichotomized scoring analysis was defined as demonstrating at least weak intensity staining (red box)
Tau expression as a predictive biomarker for taxane benefit
Exploratory analysis of the main TACT trial suggested benefit from docetaxel might be related to ER negativity and HER2 positivity, with a suggestion of docetaxel benefit in node-positive patients with ER-negative/HER2-positive disease (HR 0.7 (95 % CI 0.49–1.00)). Dichotomised cut-offs for Tau and ER status (i.e. positive or negative) demonstrated no significant interaction between Tau expression and efficacy of docetaxel (Fig. 4A). Although not statistically significant, patients with ER-negative/HER2-positive/Tau positive tumours appeared to exhibit an association with greater taxane benefit; contrary to the preclinical hypothesis that Tau expression induced resistance to taxanes and that taxane benefit would be enriched in Tau negative patients. These results were further validated using the ‘combined’ and the ‘Allred’ scores for Tau and ER, respectively, confirming no role of Tau as a predictive marker for taxane benefit (Fig. 4B, C).
Tau expression and docetaxel efficacy within breast cancer subgroup. Forest plots reporting on the interaction between Tau expression and efficacy of docetaxel. A by dichotomized cut off for Tau, ER and HER2. B By ‘combined’ score for Tau. C By Allred scores for ER. The solid squares are centred on the point estimate, and the horizontal line through each square represents the 95 % CI. The size of each square represents the weight of the study in the subgroup analysis. The centre of the diamond represents the summary estimate of the effect size, and the horizontal tips represent the 95 % CI. The solid vertical line corresponds to no effect, and the dashed vertical line corresponds to the summary estimate. CI confidence interval
Tau expression as a prognostic biomarker
In agreement with the NSABP B-28 trial analysis, Tau expression (both dichotomised and combined scoring) was seen to be a prognostic factor on univariate analysis associated with an improved DFS, independent of the treatment groups (p < 0.001, Fig. 5A, B). Among patients with ER-positive tumours (n = 1,596), patients with Tau positive disease (n = 1,069) had an improved DFS (Fig. 5C) compared with Tau negative tumours (p = 0.02). Among the patients with ER-negative cancers (n = 887), Tau expression had no prognostic value (Fig. 5C). Tau may appear to be prognostic in ER-positive patients due to its association with degrees of ER positivity, rather than being a truly independent factor. To test this we used quantitative ER in the analysis, the weak prognostic effect of Tau did not remain in the proportional hazards model when considering ER as an ordinal variable, however, there was some evidence of a prognostic effect with regard to events occurring early in follow up (Fig. 5D and see multivariable analysis (Table 2).
Univariate analyses of disease-free survival (DFS). Kaplan–Meier curves are shown. A DFS according to dichotomized Tau status and treatment subgroups: Blue line control/Tau negative, green line FEC-T/Tau negative, red line control/Tau positive and orange line FEC-T/Tau positive. B DFS according to combined Tau status: Blue line low Tau, red line intermediate Tau, green line high Tau. C DFS according to dichotomized ER and Tau subgroups: blue line ER and Tau negative, green line ER positive and Tau negative, red line ER negative and Tau positive and orange line ER and Tau positive. D DFS according to ER Allred scoring and dichotomized Tau subgroups. Cases with negative and high Allred ER scores only are depicted: blue line ER 0–2 and Tau negative, green line ER 7–8 and Tau negative, red line ER 0–2 and Tau positive; orange line ER 7–8 and Tau positive. Control = 8*FEC60 or 4*E-4*CMF. DFS disease-free survival
Tau and PR expression
Like Tau, PR is an ER-regulated gene and, as studies indicate Tau expression is perhaps a reflection of ER downstream function, we therefore, hypothesized that Tau expression would correlate strongly with PR expression. As described, central PR status correlated positively with Tau expression (Table 1). Dichotomised cut-offs for Tau and PR status (i.e. positive or negative), demonstrated no significant interaction between Tau expression and efficacy of docetaxel in patients within the PR-positive and negative subsets (Fig. 6A). No prognostic effect of Tau was observed amongst PR subgroups (Fig. 6B).
Tau and PR expression. A Forest plots reporting on the interaction between Tau and PR expression. B Kaplan–Meier curve reporting on the DFS according to PR Allred scoring and dichotomized Tau subgroups. Cases with negative and high Allred PR scores only are depicted: blue line PR 0–2 and Tau negative, green line PR 7–8 and Tau negative, red line PR 0–2 and Tau positive and orange line PR 7–8 and Tau positive. DFS disease-free survival
Multivariable analyses
Multivariable analyses were performed separately for ER-positive and ER-negative cancers (Table 2). Tau had no prognostic value in either of these groups when proportional hazards throughout follow up were assumed. Investigation of time-dependent effects in the multivariable model suggested that high Tau expression may be associated with a time-dependent effect relative to the low-expression category. In the ER-positive group, the risk of an event in the high Tau category relative to the low category is approximately a half in the first 2 years, but this effect has largely disappeared in 5 years; this can be seen in the early separation of Tau negative vs Tau positive curves in Fig. 5C, D, the curves separate within the first 2 years, but thereafter, the risk of an event is similar in both groups.
Discussion
In this study, we investigated the predictive and prognostic value of Tau in patients enrolled into the TACT trial examining the role of taxane benefit as adjuvant therapy in early breast cancer. Clinical studies evaluating Tau as a biomarker for prognostic and treatment predictive effects have been conflicting; this may relate to their design. Although dichotomization of quantitative variables is a common approach in clinical research, it may be seen as introducing an extreme form of rounding, with an inevitable loss of information and power [31]. There is a risk of underestimating the extent of variation in outcome between groups, as individuals close to, but on opposite sides of, the cut-point are characterized as being very different rather than very similar. Dichotomization has also been shown to increase the probability of false positive results [32]. Within our study analyses using both dichotomized and ordinal parameters for Tau, ER and PR analyses were, therefore, performed.
We observed no evidence for a role of Tau as a predictive marker of taxane benefit. Contrary to the hypothesis that taxane benefit would be enriched in Tau negative tumours, the only group with (non-significantly) reduced event rate in the taxane group were the HER2- and Tau positive subgroup. This is now the second large adjuvant study which, despite our studies use of both dichotomized and ordinal parameters, fails to show an association between Tau and response to taxane therapy; suggesting that measurement of Tau does not aid in clinical decision making to select optimal use of taxanes. Thus, the preclinical observations that high Tau expression confers selective resistance to paclitaxel in breast cancer cell lines remains largely unsubstantiated in clinical trials [16, 17]. In clinical studies, it is likely that other molecular mechanisms relating to chemotherapy resistance override the importance of Tau as a predictive biomarker for response to taxane therapy. Mechanisms of resistance are complex and are likely to be multiple, it may, therefore, be difficult to detect individual molecular markers that predict cytotoxicity from microtubule stabilizing agents like taxanes from amongst the competing mechanisms. In addition, because of the high level of biological, molecular and genetic heterogeneity in breast cancer, the use of a single marker is unlikely to be sufficiently specific and reliable. It is also worth noting that low Tau expression is closely associated with ER negativity, high histological grade and HER2 positivity, which might explain the earlier observations that low Tau was associated with higher pCR rates in a group with the worst prognosis.
Within our study, Tau expression was seen to be a prognostic factor associated with improved DFS, when all breast cancer patients are analysed together on univariate analysis. Tau is an ER-regulated gene with expression induced by both oestrogen and tamoxifen in vitro. The improved DFS amongst the Tau positive (i.e. high expression) patients in the trial population is likely to be a surrogate for endocrine sensitive, ER-positive tumours in which the relative sensitivity or resistance between individual chemotherapy agents is of limited relevance. Stratification by ER status confirmed that Tau expression had no prognostic value in ER-negative tumours. The good prognostic effect seen among patients with ER-positive cancers, however, was weakened, when applying ER as an ordinal variable and where the added effect of Tau is minor compared with the prognostic effect of quantitative ER in a group of patients treated with appropriate endocrine therapy. Multivariable analyses performed separately for ER-positive and ER-negative cancers also indicated that Tau had limited prognostic value in either of these groups beyond possibly identifying a subgroup of ER-positive patients with good prognosis during early follow up. PR, another oestrogen-regulated gene, approached statistical significance in the ER-positive group. Importantly, our conclusions apply to an essentially higher risk patient population who had been selected for chemotherapy and randomization to a taxane-based regimen in the TACT trial.
Disparities between RNA and protein expression, as well as the techniques used to assess these parameters [18, 22, 23, 26] may also have contributed to lack of agreement between studies. In separate clinical studies, RT-PCR and different immunohistochemistry approaches have been used to assess Tau expression. Tau has six isoforms that are spliced from a single gene and in vitro studies indicate that expression of Tau protein isoforms less than 70 kDa have the most influence on sensitivity to taxanes [24] Therefore, analysis of Tau mRNA expression may not be an appropriate for examining the utility of Tau as a predictor of taxane sensitivity. Furthermore, the status of the expression of different Tau protein isoforms is important in determining sensitivity to taxanes, but immunohistochemistry cannot, at present, be used to identify separate isoforms [24]. The antibody used in our and other studies [16, 20], recognizes both non-phosphorylated and phosphorylated forms of Tau proteins (45–68 kD). There may be value in the examination of individual Tau isoform expression in breast cancer tissues to further inform how Tau may be related to other aspects of the biology to response to taxanes.
Identification of robust biomarkers capable of identifying women with a high likelihood of response to taxanes would represent a significant advance in breast cancer research. When Tau was first proposed as a potential biomarker to discriminate between taxane sensitivity and resistance, there was great optimism that it may offer clinical utility in improving patient care. This report from the adjuvant taxane trial TACT, along with those from the GeparTrio, NSABP and HECOG trials confirms that there is no clinical application for Tau and demonstrates again the perils of relying on a single biomarker to report the complexity of drug resistance in relation to clinical outcome. This is disappointing, but recent advances in genomic and proteomic technologies are likely to drive the development of novel multi-component companion diagnostic biomarkers for the taxane class of drugs and may prove to be more fruitful in the future.
References
De Laurentiis M, Cancello G, D’Agostino D, Giuliano M, Giordano A, Montagna E, Lauria R, Forestieri V, Esposito A, Silvestro L et al (2008) Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol 26(1):44–53
Bria E, Nistico C, Cuppone F, Carlini P, Ciccarese M, Milella M, Natoli G, Terzoli E, Cognetti F, Giannarelli D (2006) Benefit of taxanes as adjuvant chemotherapy for early breast cancer: pooled analysis of 15,500 patients. Cancer 106(11):2337–2344
Nowak AK, Wilcken NR, Stockler MR, Hamilton A, Ghersi D (2004) Systematic review of taxane-containing versus non-taxane-containing regimens for adjuvant and neoadjuvant treatment of early breast cancer. Lancet Oncol 5(6):372–380
Trudeau M, Charbonneau F, Gelmon K, Laing K, Latreille J, Mackey J, McLeod D, Pritchard K, Provencher L, Verma S (2005) Selection of adjuvant chemotherapy for treatment of node-positive breast cancer. Lancet Oncol 6(11):886–898
Trudeau M, Sinclair SE, Clemons M (2005) Neoadjuvant taxanes in the treatment of non-metastatic breast cancer: a systematic review. Cancer Treat Rev 31(4):283–302
Early Breast Cancer Trialists’ Collaborative G, Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, Cutter D, Darby S, McGale P et al (2012) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379(9814):432–444
Ellis P, Barrett-Lee P, Johnson L, Cameron D, Wardley A, O’Reilly S, Verrill M, Smith I, Yarnold J, Coleman R et al (2009) Sequential docetaxel as adjuvant chemotherapy for early breast cancer (TACT): an open-label, phase III, randomised controlled trial. Lancet 373(9676):1681–1692
Bliss JM, Ellis P, Kilburn L, Bartlett J, Bloomfield D, Cameron D, Canney P, Coleman RE, Dowsett M, Earl H, Verril M, Wardley A, Yarnold J, Ahern R, Atkins N, Fletcher M, McLinden M, Barrett-Lee P (2012) Mature analysis of UK Taxotere as Adjuvant Chemotherapy (TACT) trial (CRUK 01/001); effects of treatment and characterisation of patterns of breast cancer relapse. Cancer Res 72(24):1–608
Palmieri C, Jones A (2012) The 2011 EBCTCG polychemotherapy overview. Lancet 379(9814):390–392
Esteva FJ, Valero V, Pusztai L, Boehnke-Michaud L, Buzdar AU, Hortobagyi GN (2001) Chemotherapy of metastatic breast cancer: what to expect in 2001 and beyond. Oncologist 6(2):133–146
Crown J, O’Leary M, Ooi WS (2004) Docetaxel and paclitaxel in the treatment of breast cancer: a review of clinical experience. Oncologist 9(Suppl 2):24–32
Felgner H, Frank R, Biernat J, Mandelkow EM, Mandelkow E, Ludin B, Matus A, Schliwa M (1997) Domains of neuronal microtubule-associated proteins and flexural rigidity of microtubules. J Cell Biol 138(5):1067–1075
Kar S, Fan J, Smith MJ, Goedert M, Amos LA (2003) Repeat motifs of tau bind to the insides of microtubules in the absence of taxol. EMBO J 22(1):70–77
Cassimeris L, Spittle C (2001) Regulation of microtubule-associated proteins. Int Rev Cytol 210:163–226
Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW (1975) A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA 72(5):1858–1862
Rouzier R, Rajan R, Wagner P, Hess KR, Gold DL, Stec J, Ayers M, Ross JS, Zhang P, Buchholz TA et al (2005) Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci USA 102(23):8315–8320
Wagner P, Wang B, Clark E, Lee H, Rouzier R, Pusztai L (2005) Microtubule Associated Protein (MAP)-Tau: a novel mediator of paclitaxel sensitivity in vitro and in vivo. Cell Cycle 4(9):1149–1152
Andre F, Hatzis C, Anderson K, Sotiriou C, Mazouni C, Mejia J, Wang B, Hortobagyi GN, Symmans WF, Pusztai L (2007) Microtubule-associated protein-tau is a bifunctional predictor of endocrine sensitivity and chemotherapy resistance in estrogen receptor-positive breast cancer. Clin Cancer Res 13(7):2061–2067
Tanaka S, Nohara T, Iwamoto M, Sumiyoshi K, Kimura K, Takahashi Y, Tanigawa N (2009) Tau expression and efficacy of paclitaxel treatment in metastatic breast cancer. Cancer Chemother Pharmacol 64(2):341–346
Shao YY, Kuo KT, Hu FC, Lu YS, Huang CS, Liau JY, Lee WC, Hsu C, Kuo WH, Chang KJ et al (2010) Predictive and prognostic values of tau and ERCC1 in advanced breast cancer patients treated with paclitaxel and cisplatin. Jpn J Clin Oncol 40(4):286–293
Rody A, Karn T, Gatje R, Ahr A, Solbach C, Kourtis K, Munnes M, Loibl S, Kissler S, Ruckhaberle E et al (2007) Gene expression profiling of breast cancer patients treated with docetaxel, doxorubicin, and cyclophosphamide within the GEPARTRIO trial: HER-2, but not topoisomerase II alpha and microtubule-associated protein tau, is highly predictive of tumor response. Breast 16(1):86–93
Pentheroudakis G, Kalogeras KT, Wirtz RM, Grimani I, Zografos G, Gogas H, Stropp U, Pectasides D, Skarlos D, Hennig G et al (2009) Gene expression of estrogen receptor, progesterone receptor and microtubule-associated protein Tau in high-risk early breast cancer: a quest for molecular predictors of treatment benefit in the context of a Hellenic Cooperative Oncology Group trial. Breast Cancer Res Treat 116(1):131–143
Pusztai L, Jeong JH, Gong Y, Ross JS, Kim C, Paik S, Rouzier R, Andre F, Hortobagyi GN, Wolmark N et al (2009) Evaluation of microtubule-associated protein-Tau expression as a prognostic and predictive marker in the NSABP-B 28 randomized clinical trial. J Clin Oncol 27(26):4287–4292
Ikeda H, Taira N, Hara F, Fujita T, Yamamoto H, Soh J, Toyooka S, Nogami T, Shien T, Doihara H et al (2010) The estrogen receptor influences microtubule-associated protein tau (MAPT) expression and the selective estrogen receptor inhibitor fulvestrant downregulates MAPT and increases the sensitivity to taxane in breast cancer cells. Breast Cancer Res 12(3):R43
Dumontet C, Krajewska M, Treilleux I, Mackey JR, Martin M, Rupin M, Lafanechere L, Reed JC (2010) BCIRG 001 molecular analysis: prognostic factors in node-positive breast cancer patients receiving adjuvant chemotherapy. Clin Cancer Res 16(15):3988–3997
Baquero MT, Lostritto K, Gustavson MD, Bassi KA, Appia F, Camp RL, Molinaro AM, Harris LN, Rimm DL (2011) Evaluation of prognostic and predictive value of microtubule associated protein tau in two independent cohorts. Breast Cancer Res 13(5):R85
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2005) Statistics Subcommittee of the NCIEWGoCD: reporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer 93(4):387–391
Bartlett JM, A’Hern R, Piper T, Ellis IO, Dowsett M, Mallon EA, Cameron DA, Johnston S, Bliss JM, Ellis P et al (2013) Phosphorylation of AKT pathway proteins is not predictive of benefit of taxane therapy in early breast cancer. Breast Cancer Res Treat 138(3):773–781
Bartlett JM, Ellis IO, Dowsett M, Mallon EA, Cameron DA, Johnston S, Hall E, A’Hern R, Peckitt C, Bliss JM et al (2007) Human epidermal growth factor receptor 2 status correlates with lymph node involvement in patients with estrogen receptor (ER) negative, but with grade in those with ER-positive early-stage breast cancer suitable for cytotoxic chemotherapy. J Clin Oncol 25(28):4423–4430
Harvey JM, Clark GM, Osborne CK, Allred DC (1999) Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 17(5):1474–1481
MacCallum RC, Zhang S, Preacher KJ, Rucker DD (2002) On the practice of dichotomization of quantitative variables. Psychol Methods 7(1):19–40
Austin PC, Brunner LJ (2004) Inflation of the type I error rate when a continuous confounding variable is categorized in logistic regression analyses. Stat Med 23(7):1159–1178
Acknowledgments
The TACT trial is jointly funded by Cancer Research UK (CRUK/01/001) and educational Grants from Aventis, Roche and Pfizer. Storage, construction of microarrays and tissue HER2 testing were funded by an educational grant from Roche. We would like to thank all the patients who agreed to enter the TACT trial and donate their tumour tissue for TransTACT. This work was supported by the Experimental Cancer Medicine Centre Initiative, which is jointly funded by Cancer Research UK, the National Institute for Health Research in England and the Departments of Health for Scotland, Wales and Northern Ireland. We acknowledge support from the NIHR RM/ICR Biomedical Research Centre. Patient tissue samples were provided by Guy’s and St Thomas’ Breast Tissue and Data Bank, which is supported by the Department of Health via the National Institute for Health Research and comprehensive Biomedical Research Centre award. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. We would like to acknowledge the funding to Dr Sheeba Irshad from Breakthrough Breast Cancer and the Sarah Greene Tribute Fund. Professor Andrew Tutt is funded by the Research Oncology and Breakthrough Breast Cancer funds.
Conflict of interest
No conflicts of interest.
Ethical standards
The experiments comply with the current laws of the United Kingdom.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Irshad, S., Gillett, C., Pinder, S.E. et al. Assessment of microtubule-associated protein (MAP)-Tau expression as a predictive and prognostic marker in TACT; a trial assessing substitution of sequential docetaxel for FEC as adjuvant chemotherapy for early breast cancer. Breast Cancer Res Treat 144, 331–341 (2014). https://doi.org/10.1007/s10549-014-2855-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-2855-4