Abstract
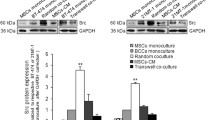
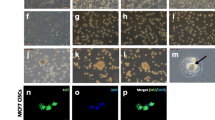
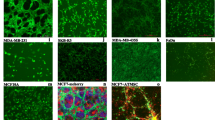
Bone marrow-derived mesenchymal stem cells (MSCs) are known to specifically migrate to and engraft at tumour sites. Understanding interactions between cancer cells and MSCs has become fundamental to determining whether MSC-tumour interactions should be harnessed for delivery of therapeutic agents or considered a target for intervention. Breast Cancer Cell lines (MDA-MB-231, T47D & SK-Br3) were cultured alone or on a monolayer of MSCs, and retrieved using epithelial specific magnetic beads. Alterations in expression of 90 genes associated with breast tumourigenicity were analysed using low-density array. Expression of markers of epithelial–mesenchymal transition (EMT) and array results were validated using RQ-PCR. Co-cultured cells were analysed for changes in protein expression, growth pattern and morphology. Gene expression and proliferation assays were also performed on indirect co-cultures. Following direct co-culture with MSCs, breast cancer cells expressed elevated levels of oncogenes (NCOA4, FOS), proto-oncogenes (FYN, JUN), genes associated with invasion (MMP11), angiogenesis (VEGF) and anti-apoptosis (IGF1R, BCL2). However, universal downregulation of genes associated with proliferation was observed (Ki67, MYBL2), and reflected in reduced ATP production in response to MSC-secreted factors. Significant upregulation of EMT specific markers (N-cadherin, Vimentin, Twist and Snail) was also observed following co-culture with MSCs, with a reciprocal downregulation in E-cadherin protein expression. These changes were predominantly cell contact mediated and appeared to be MSC specific. Breast cancer cell morphology and growth pattern also altered in response to MSCs. MSCs may promote breast cancer metastasis through facilitation of EMT.





Similar content being viewed by others
References
Jemal A, Siegel R, Ward E et al (2008) Cancer statistics, 2008. CA Cancer J Clin 58:71–96
Coleman RE, Rubens RD (1987) The clinical course of bone metastases from breast cancer. Br J Cancer 55:61–66
Espey DK, Wu XC, Swan J et al (2007) Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer 110:2119–2152
Bhowmick NA, Moses HL (2005) Tumor-stroma interactions. Curr Opin Genet Dev 15:97–101
Hu M, Polyak K (2008) Molecular characterisation of the tumour microenvironment in breast cancer. Eur J Cancer 44:2760–2765
Pittenger MF, Mackay AM, Beck SC et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Hombauer H, Minguell JJ (2000) Selective interactions between epithelial tumour cells and bone marrow mesenchymal stem cells. Br J Cancer 82:1290–1296
Fierro FA, Sierralta WD, Epunan MJ et al (2004) Marrow-derived mesenchymal stem cells: role in epithelial tumor cell determination. Clin Exp Metastasis 21:313–319
Sasser AK, Mundy BL, Smith KM et al (2007) Human bone marrow stromal cells enhance breast cancer cell growth rates in a cell line-dependent manner when evaluated in 3D tumor environments. Cancer Lett 254:255–264
Chen J, Zhang ZG, Li Y et al (2003) Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res 92:692–699
Spaeth E, Klopp A, Dembinski J et al (2008) Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther 15:730–738
Dvorak HF (1986) Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315:1650–1659
Kumar S, Chanda D, Ponnazhagan S (2008) Therapeutic potential of genetically modified mesenchymal stem cells. Gene Ther 15:711–715
Karnoub AE, Dash AB, Vo AP et al (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449:557–563
Dwyer RM, Potter-Beirne SM, Harrington KA et al (2007) Monocyte chemotactic protein-1 (MCP-1) secreted by primary breast tumors stimulates migration of mesenchymal stem cells (MSCs). Clin Cancer Res 13:5020–5027
Molloy AP, Martin FT, Dwyer RM et al (2009) Mesenchymal stem cell secretion of chemokines during differentiation into osteoblasts, and their potential role in mediating interactions with breast cancer cells. Int J Cancer 124:326–332
Nakaya Y, Sheng G (2008) Epithelial to mesenchymal transition during gastrulation: an embryological view. Dev Growth Differ 50:755–766
Thiery JP, Sleeman JP (2006) Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 7:131–142
Brabletz T, Jung A, Spaderna S et al (2005) Opinion: migrating cancer stem cells––an integrated concept of malignant tumour progression. Nat Rev Cancer 5:744–749
Sarrio D, Rodriguez-Pinilla SM, Hardisson D et al (2008) Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res 68:989–997
Mani SA, Guo W, Liao MJ et al (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133:704–715
Barry FP, Murphy JM (2004) Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol 36:568–584
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408
Woelfle U, Breit E, Pantel K (2005) Influence of immunomagnetic enrichment on gene expression of tumor cells. J Transl Med 3:12
Corcoran KE, Trzaska KA, Fernandes H et al (2008) Mesenchymal stem cells in early entry of breast cancer into bone marrow. PLoS ONE 3:e2563
Molloy AP, Martin FT, Dwyer RM et al (2008) Mesenchymal stem cell secretion of chemokines during differentiation into osteoblasts, and their potential role in mediating interactions with breast cancer cells. Int J Cancer 124:326
Gelmini S, Mangoni M, Serio M et al (2008) The critical role of SDF-1/CXCR4 axis in cancer and cancer stem cells metastasis. J Endocrinol Invest 31:809–819
Eferl R, Wagner EF (2003) AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer 3:859–868
Pinilla SM, Honrado E, Hardisson D et al (2006) Caveolin-1 expression is associated with a basal-like phenotype in sporadic and hereditary breast cancer. Breast Cancer Res Treat 99:85–90
De Wever O, Pauwels P, De Craene B et al (2008) Molecular and pathological signatures of epithelial-mesenchymal transitions at the cancer invasion front. Histochem Cell Biol 130:481–494
Thomas PA, Kirschmann DA, Cerhan JR et al (1999) Association between keratin and vimentin expression, malignant phenotype, and survival in postmenopausal breast cancer patients. Clin Cancer Res 5:2698–2703
McInroy L, Maatta A (2007) Down-regulation of vimentin expression inhibits carcinoma cell migration and adhesion. Biochem Biophys Res Commun 360:109–114
Galliher AJ, Schiemann WP (2006) Beta3 integrin and Src facilitate transforming growth factor-beta mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res 8:R42
Mercado-Pimentel ME, Runyan RB (2007) Multiple transforming growth factor-beta isoforms and receptors function during epithelial-mesenchymal cell transformation in the embryonic heart. Cells Tissues Organs 185:146–156
Derynck R, Akhurst RJ, Balmain A (2001) TGF-beta signaling in tumor suppression and cancer progression. Nat Genet 29:117–129
Han G, Lu SL, Li AG et al (2005) Distinct mechanisms of TGF-beta1-mediated epithelial-to-mesenchymal transition and metastasis during skin carcinogenesis. J Clin Invest 115:1714–1723
Mercurio AM, Lipscomb EA, Bachelder RE (2005) Non-angiogenic functions of VEGF in breast cancer. J Mammary Gland Biol Neoplasia 10:283–290
Wanami LS, Chen HY, Peiro S et al (2008) Vascular endothelial growth factor-A stimulates Snail expression in breast tumor cells: implications for tumor progression. Exp Cell Res 314:2448–2453
Enciso JM, Gratzinger D, Camenisch TD et al (2003) Elevated glucose inhibits VEGF-A-mediated endocardial cushion formation: modulation by PECAM-1 and MMP-2. J Cell Biol 160:605–615
Acknowledgements
This study was supported by funding from the National Breast Cancer Research Institute (NBCRI), a Royal College of Surgeons in Ireland (RCSI) Surgical Research Grant (F.T. Martin), a Health Research Board Project Grant (R.M. Dwyer), and a Science Foundation Ireland CSET award (J.M. Murphy, F.P. Barry and T. O’Brien).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martin, F.T., Dwyer, R.M., Kelly, J. et al. Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res Treat 124, 317–326 (2010). https://doi.org/10.1007/s10549-010-0734-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-0734-1