Abstract
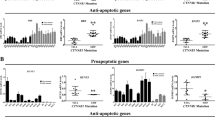
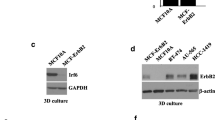
Dickkopf-1 (DKK-1) is a secreted inhibitor of the Wnt signaling pathway. We previously identified DKK-1 as a candidate tumor suppressor and demonstrated that ectopic expression of the DKK-1 suppressed the tumorigenicity of HeLa cells in vitro and in vivo. Since suppression of tumorigenicity of HeLa cells by DKK-1 overexpression was not mediated by effects on β-catenin dependent transcription, we hypothesized that DKK-1 might also inhibit tumorigenicity of breast carcinoma cell lines lacking an activated canonical Wnt pathway. In the present study we show that ectopic expression of DKK-1 in various breast cancer cell lines resulted in a change in the cell phenotype, increased sensitivity to apoptosis, inhibition of anchorage independent growth in vitro, and suppression of tumorigenicity in vivo. Consistent with known effects of DKK-1 on the canonical Wnt signaling pathway, ectopic expression of DKK-1 in breast carcinoma cells was associated with increased phosphorylation and degradation of β-catenin. However, none of the breast tumor cells used in this study showed detectable levels of β-catenin dependent activation of TCF/Lef promoter activity measured by reporter constructs. Consistent with the results of these transient transfection assays, we were unable to demonstrate the expected β-catenin dependent, TCF/Lef mediated inhibition of cyclin D1 and c-myc gene transcription in breast cells overexpressing DKK-1. However, we found that cells with DKK-1 overexpression have increased activity of CamKII pathway. Overexpression of the constitutively active form of CamKII (T286D) resulted in inhibition of breast cancer cell tumorigenicity. Thus, our study supports the hypothesis that DKK-1 mediated tumor suppressor effect is independent of β-catenin dependent transcription and identified the CamKII pathway that contributes into DKK-1 signaling.






Similar content being viewed by others
References
Fedi P, Bafico A, Nieto Soria A et al (1999) Isolation and biochemical characterization of the human Dkk-1 homologue, a novel inhibitor of mammalian Wnt signaling. J Biol Chem 274(27):19465–19472
Bafico A, Liu G, Yaniv A et al (2001) Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol 3(7):683–686
Mao B, Wu W, Davidson G et al (2002) Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature 417(6889):664–667
Mao B, Wu W, Li Y et al (2001) LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature 411(6835):321–325
Clevers H (2004) Wnt breakers in colon cancer. Cancer Cell 5(1):5–6
He X, Semenov M, Tamai K et al (2004) LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development 131(8):1663–1677
Polakis P (2000) Wnt signaling and cancer. Genes Dev 14(15):1837–1851
Satoh S, Daigo Y, Furukawa Y et al (2000) AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet 24(3):245–250
Akiyama T (2000) Wnt/beta-catenin signaling. Cytokine Growth Factor Rev 11(4):273–282
Buendia MA (2000) Genetics of hepatocellular carcinoma. Semin Cancer Biol 10(3):185–200
Semba S, Yamakawa M, Sasano H (2001) The cadherin-catenin superfamily in endocrine tumors. Endocr Pathol 12(1):1–13
Candidus S, Bischoff P, Becker KF et al (1996) No evidence for mutations in the alpha- and beta-catenin genes in human gastric and breast carcinomas. Cancer Res 56(1):49–52
Jonsson M, Borg A, Nilbert M et al (2000) Involvement of adenomatous polyposis coli (APC)/beta-catenin signalling in human breast cancer. Eur J Cancer 36(2):242–248
Schlosshauer PW, Brown SA, Eisinger K et al (2000) APC truncation and increased beta-catenin levels in a human breast cancer cell line. Carcinogenesis 21(7):1453–1456
Nusse R, Varmus HE (1982) Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31(1):99–109
Nusse R, Varmus HE (1992) Wnt genes. Cell 69(7):1073–1087
Chung GG, Zerkowski MP, Ocal IT et al (2004) Beta-catenin and p53 analyses of a breast carcinoma tissue microarray. Cancer 100(10):2084–2092
Lin SY, Xia W, Wang JC et al (2000) Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci USA 97(8):4262–4266
Aguilera O, Fraga MF, Ballestar E et al (2006) Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene 25(29):4116–4121
Bafico A, Liu G, Goldin L et al (2004) An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell 6(5):497–506
Hoang BH, Kubo T, Healey JH et al (2004) Dickkopf 3 inhibits invasion and motility of Saos-2 osteosarcoma cells by modulating the Wnt-beta-catenin pathway. Cancer Res 64(8):2734–2739
Gonzalez-Sancho JM, Aguilera O, Garcia JM et al (2005) The Wnt antagonist DICKKOPF-1 gene is a downstream target of beta-catenin/TCF and is downregulated in human colon cancer. Oncogene 24(6):1098–1103
Kobayashi K, Ouchida M, Tsuji T et al (2002) Reduced expression of the REIC/Dkk-3 gene by promoter-hypermethylation in human tumor cells. Gene 282(1–2):151–158
Kuphal S, Lodermeyer S, Bataille F et al (2006) Expression of Dickkopf genes is strongly reduced in malignant melanoma. Oncogene 25(36):5027–5036
Yamaguchi Y, Itami S, Watabe H et al (2004) Mesenchymal–epithelial interactions in the skin: increased expression of dickkopf1 by palmoplantar fibroblasts inhibits melanocyte growth and differentiation. J Cell Biol 165(2):275–285
Lee AY, He B, You L et al (2004) Dickkopf-1 antagonizes Wnt signaling independent of beta-catenin in human mesothelioma. Biochem Biophys Res Commun 323(4):1246–1250
Mikheev AM, Mikheeva SA, Liu B et al (2004) A functional genomics approach for the identification of putative tumor suppressor genes: Dickkopf-1 as suppressor of HeLa cell transformation. Carcinogenesis 25(1):47–59
Ishitani T, Kishida S, Hyodo-Miura J et al (2003) The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol Cell Biol 23(1):131–139
Chan TA, Wang Z, Dang LH et al (2002) Targeted inactivation of CTNNB1 reveals unexpected effects of beta-catenin mutation. Proc Natl Acad Sci USA 99(12):8265–8270
Fan S, Smith ML, Rivet DJ 2nd et al (1995) Disruption of p53 function sensitizes breast cancer MCF-7 cells to cisplatin and pentoxifylline. Cancer Res 55(8):1649–1654
Morvan F, Boulukos K, Clement-Lacroix P et al (2006) Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Mineral Res 21(6):934–945
Mikheev AM, Mikheeva SA, Rostomily R et al (2007) Dickkopf-1 activates cell death in MDA-MB435 melanoma cells. Biochem Biophys Res Commun 352(3):675–680
Wang J, Shou J, Chen X (2000) Dickkopf-1, an inhibitor of the Wnt signaling pathway, is induced by p53. Oncogene 19(14):1843–1848
Karim R, Tse G, Putti T et al (2004) The significance of the Wnt pathway in the pathology of human cancers. Pathology 36(2):120–128
Kirikoshi H, Katoh M (2002) Expression of WNT7A in human normal tissues and cancer, and regulation of WNT7A and WNT7B in human cancer. Int J Oncol 21(4):895–900
Kirikoshi H, Katoh M (2002) Expression and regulation of WNT10B in human cancer: up-regulation of WNT10B in MCF-7 cells by beta-estradiol and down-regulation of WNT10B in NT2 cells by retinoic acid. Int J Mol Med 10(4):507–511
van de Wetering M, Barker N, Harkes IC et al (2001) Mutant E-cadherin breast cancer cells do not display constitutive Wnt signaling. Cancer Res 61(1):278–284
Pukrop T, Klemm F, Hagemann T et al (2006) Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci USA 103(14):5454–5459
Kuhl M (2004) The WNT/calcium pathway: biochemical mediators, tools and future requirements. Front Biosci 9:967–974
Kremenevskaja N, von Wasielewski R, Rao AS et al (2005) Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene 24(13):2144–2154
Topol L, Jiang X, Choi H et al (2003) Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol 162(5):899–908
Ouko L, Ziegler TR, Gu LH et al (2004) Wnt11 signaling promotes proliferation, transformation, and migration of IEC6 intestinal epithelial cells. J Biol Chem 279(25):26707–26715
Weeraratna AT, Jiang Y, Hostetter G et al (2002) Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 1(3):279–288
Shulewitz M, Soloviev I, Wu T et al (2006) Repressor roles for TCF-4 and Sfrp1 in Wnt signaling in breast cancer. Oncogene 25(31):4361–4369
Acknowledgements
We thank Dr. Randall Moon for valuable discussion. This research was supported by Public Health Services, with NIH grants to the NIEHS sponsored FHCRC/UW Toxicogenomics Research Consortium, Grant # NIEHS U19ES011387, and the NIEHS sponsored UW Center for Ecogenetics and Environmental Health, Grant #: NIEHS P30ES07033, by an IDEA grant from the U.S. Army Medical Research and Materiel Command, Grant # DAMD17-98-1-8086 and NIH/NINDS training grant NS0007144. J.V.R. is supported by the University of Washington Anesthesiology Departmental Research Fund.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mikheev, A.M., Mikheeva, S.A., Maxwell, JP. et al. Dickkopf-1 mediated tumor suppression in human breast carcinoma cells. Breast Cancer Res Treat 112, 263–273 (2008). https://doi.org/10.1007/s10549-007-9867-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9867-2