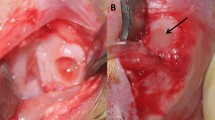
Abstract
Evaluate parylene scaffold feasibility in cartilage lesion treatment, introducing a novel paradigm combining a reparative and superficial reconstructive procedure. Fifteen rabbits were used. All animals had both knees operated and the same osteochondral lesion model was created bilaterally. The parylene scaffold was implanted in the right knee, and the left knee of the same animal was used as control. The animals were euthanized at different time points after surgery: four animals at three weeks, three animals at six weeks, four animals at nine weeks, and four animals at 12 weeks. Specimens were analyzed by International Cartilage Repair Society (ICRS) macroscopic evaluation, modified Pineda histologic evaluation of cartilage repair, and collagen II immunostaining. Parylene knees were compared to its matched contra-lateral control knees of the same animal using the Wilcoxon matched-pairs signed rank. ICRS mean ± SD values for parylene versus control, three, six, nine and twelve weeks, respectively: 7.83 ± 1.85 versus 4.42 ± 1.08, p = 0.0005; 10.17 ± 1.17 versus 6.83 ± 1.17, p = 0.03; 10.89 ± 0.60 versus 7.33 ± 2.18, p = 0.007; 10.67 ± 0.78 versus 7.83 ± 3.40, p = 0.03. Modified Pineda mean ± SD values for parylene versus control, six, nine and twelve weeks, respectively: 3.37 ± 0.87 versus 6.94 ± 1.7, p < 0.0001; 5.73 ± 2.05 versus 6.41 ± 1.7, p = 0.007; 3.06 ± 1.61 versus 6.52 ± 1.51, p < 0.0001. No inflammation was seen. Parylene implanted knees demonstrated higher collagen II expression via immunostaining in comparison to the control knees. Parylene scaffolds are a feasible option for cartilage lesion treatment and the combination of a reparative to a superficial reconstructive procedure using parylene scaffolds led to better results than the reparative procedure alone.








Similar content being viewed by others
References
A.B. Adesida, A. Mulet-Sierra, N.M. Jomha, Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res Ther 3, 9 (2012)
S. Anders, M. Volz, H. Frick, J. Gellissen, A randomized, controlled trial comparing autologous matrix-induced Chondrogenesis (AMIC(R)) to Microfracture: Analysis of 1- and 2-year follow-up data of 2 centers. Open Orthop J 7, 133–143 (2013)
D.E. Anderson, B.D. Markway, D. Bond, H.E. McCarthy, B. Johnstone, Responses to altered oxygen tension are distinct between human stem cells of high and low chondrogenic capacity. Stem Cell Res Ther 7, 154 (2016)
B.B. Christensen, C.B. Foldager, O.M. Hansen, A.A. Kristiansen, D.Q. Le, A.D. Nielsen, J.V. Nygaard, C.E. Bunger, M. Lind, A novel nano-structured porous polycaprolactone scaffold improves hyaline cartilage repair in a rabbit model compared to a collagen type I/III scaffold: In vitro and in vivo studies. Knee Surg. Sports Traumatol. Arthrosc. 20, 1192–1204 (2012)
C.R. Chu, M. Szczodry, S. Bruno, Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev 16, 105–115 (2010)
F. Cicuttini, C. Ding, A. Wluka, S. Davis, P.R. Ebeling, G. Jones, Association of cartilage defects with loss of knee cartilage in healthy, middle-age adults: A prospective study. Arthritis Rheum. 52, 2033–2039 (2005)
C. Ding, F. Cicuttini, F. Scott, H. Cooley, C. Boon, G. Jones, Natural history of knee cartilage defects and factors affecting change. Arch. Intern. Med. 166, 651–658 (2006)
B. Diniz, P. Thomas, B. Thomas, R. Ribeiro, Y. Hu, R. Brant, A. Ahuja, D. Zhu, L. Liu, M. Koss, M. Maia, G. Chader, D.R. Hinton, M.S. Humayun, Subretinal implantation of retinal pigment epithelial cells derived from human embryonic stem cells: Improved survival when implanted as a monolayer. Invest. Ophthalmol. Vis. Sci. 54, 5087–5096 (2013)
C.E. Franciozi, V.A. Tarini, R.D. Reginato, P.R. Goncalves, V.P. Medeiros, M. Ferretti, J.L. Dreyfuss, H.B. Nader, F. Faloppa, Gradual strenuous running regimen predisposes to osteoarthritis due to cartilage cell death and altered levels of glycosaminoglycans. Osteoarthr. Cartil. 21, 965–972 (2013)
G.C. Gracitelli, V.Y. Moraes, C.E. Franciozi, M.V. Luzo, J.C. Belloti, Surgical interventions (microfracture, drilling, mosaicplasty, and allograft transplantation) for treating isolated cartilage defects of the knee in adults. Cochrane Database Syst. Rev. 9, CD010675 (2016)
E.B. Hunziker, L.C. Rosenberg, Repair of partial-thickness defects in articular cartilage: Cell recruitment from the synovial membrane. J. Bone Joint Surg. Am. 78, 721–733 (1996)
T.J. Levingstone, E. Thompson, A. Matsiko, A. Schepens, J.P. Gleeson, F.J. O'Brien, Multi-layered collagen-based scaffolds for osteochondral defect repair in rabbits. Acta Biomater. 32, 149–160 (2016)
M. Lind, F. Menhert, A.B. Pedersen, The first results from the Danish ACL reconstruction registry: Epidemiologic and 2 year follow-up results from 5,818 knee ligament reconstructions. Knee Surg. Sports Traumatol. Arthrosc. 17, 117–124 (2009)
B. Lu, D. Zhu, D. Hinton, M.S. Humayun, Y.C. Tai, Mesh-supported submicron parylene-C membranes for culturing retinal pigment epithelial cells. Biomed. Microdevices 14, 659–667 (2012)
P. Mainil-Varlet, B. Van Damme, D. Nesic, G. Knutsen, R. Kandel, S. Roberts, A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II. Am. J. Sports Med. 38, 880–890 (2010)
H.J. Mankin, The response of articular cartilage to mechanical injury. J. Bone Joint Surg. Am. 64, 460–466 (1982)
V.M. Mattila, R. Sihvonen, J. Paloneva, L. Fellander-Tsai, Changes in rates of arthroscopy due to degenerative knee disease and traumatic meniscal tears in Finland and Sweden. Acta Orthop. 87, 5–11 (2016)
D.J. Moojen, D.B. Saris, K.G. Auw Yang, W.J. Dhert, A.J. Verbout, The correlation and reproducibility of histological scoring systems in cartilage repair. Tissue Eng. 8, 627–634 (2002)
C.J. O'Conor, N. Case, F. Guilak, Mechanical regulation of chondrogenesis. Stem Cell Res Ther 4, 61 (2013)
S.W. O'Driscoll, F.W. Keeley, R.B. Salter, The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. An experimental investigation in the rabbit. J. Bone Joint Surg. Am. 68, 1017–1035 (1986)
L. Peterson, T. Minas, M. Brittberg, A. Nilsson, E. Sjogren-Jansson, A. Lindahl, Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin. Orthop. Relat. Res., 212–234 (2000)
S. Pineda, A. Pollack, S. Stevenson, V. Goldberg, A. Caplan, A semiquantitative scale for histologic grading of articular cartilage repair. Acta Anat. (Basel) 143, 335–340 (1992)
A.R. Poole, T. Kojima, T. Yasuda, F. Mwale, M. Kobayashi, S. Laverty, Composition and structure of articular cartilage: A template for tissue repair. Clin. Orthop. Relat. Res., S26–S33 (2001)
S. Portron, V. Hivernaud, C. Merceron, J. Lesoeur, M. Masson, O. Gauthier, C. Vinatier, L. Beck, J. Guicheux, Inverse regulation of early and late chondrogenic differentiation by oxygen tension provides cues for stem cell-based cartilage tissue engineering. Cell. Physiol. Biochem. 35, 841–857 (2015)
B. Pouran, V. Arbabi, A.A. Zadpoor, H. Weinans, Isolated effects of external bath osmolality, solute concentration, and electrical charge on solute transport across articular cartilage. Med. Eng. Phys. 38, 1399–1407 (2016)
D.C. Rodger, A.J. Fong, W. Li, H. Ameri, A.K. Ahuja, C. Gutierrez, I. Lavrov, H. Zhong, P.R. Menon, E. Meng, J.W. Burdick, R.R. Roy, V. Reggie Edgerton, J.D. Weiland, M.S. Humayun, Y.-C. Tai, Flexible parylene-based multielectrode array technology for high-density neural stimulation and recording. Sensors Actuators B Chem. 132, 449–460 (2008)
M. Rutgers, M.J. van Pelt, W.J. Dhert, L.B. Creemers, D.B. Saris, Evaluation of histological scoring systems for tissue-engineered, repaired and osteoarthritic cartilage. Osteoarthr. Cartil. 18, 12–23 (2010)
F. Shapiro, S. Koide, M.J. Glimcher, Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J. Bone Joint Surg. Am. 75, 532–553 (1993)
G.D. Smith, J. Taylor, K.F. Almqvist, C. Erggelet, G. Knutsen, M. Garcia Portabella, T. Smith, J.B. Richardson, Arthroscopic assessment of cartilage repair: A validation study of 2 scoring systems. Arthroscopy 21, 1462–1467 (2005)
M.P. van den Borne, N.J. Raijmakers, J. Vanlauwe, J. Victor, S.N. de Jong, J. Bellemans, D.B. Saris, Society International Cartilage Repair, International cartilage repair Society (ICRS) and Oswestry macroscopic cartilage evaluation scores validated for use in autologous chondrocyte implantation (ACI) and microfracture. Osteoarthr. Cartil. 15, 1397–1402 (2007)
W. Widuchowski, J. Widuchowski, T. Trzaska, Articular cartilage defects: Study of 25,124 knee arthroscopies. Knee 14, 177–182 (2007)
Acknowledgements
This study was supported by Vangsness Research Fund from Keck School of Medicine – University of Southern California. Franciozi CE received post-doctoral scholarship from Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP (grant# 2015/08952-6) supporting his scientific activities at the University of Southern California from 2015 to 2016.
The authors would like to thank FAPESP, Andrea Moon, James D. Weiland, Yi Zhang, Ellis Troy, Doris Lee, Fernando Gallardo, Lina Flores, Luis V. Jimenez, Alex Mcintyre, Ian Jones, Andrea Zanetti, Samantha L. Brims, James Finlay, Sumanth Putta, Sean Veal, Mario Rogel, Ziri Sadai Quikano, Joshua Siyoung Lee, Cecilia Melendres, Susanne Vaage, Sheila McNeil Ingham, Roseli Paschoa, Mario Ferretti, Flavio Faloppa and Paulo Rodrigues. Without the help, support, and enthusiasm of all of these people, this work would not have been possible.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement on the welfare of animals
Ethical approval: All applicable international and institutional guidelines for the care and use of animals were followed. All procedures performed were done in accordance with the ethical standards of the institution at which the study was conducted and was approved by its Institutional Animal Care and Use Committee.
Rights and permissions
About this article
Cite this article
Franciozi, C.E.d., Vangsness, C.T., Tibone, J.E. et al. Parylene scaffold for cartilage lesion. Biomed Microdevices 19, 26 (2017). https://doi.org/10.1007/s10544-017-0170-7
Published:
DOI: https://doi.org/10.1007/s10544-017-0170-7