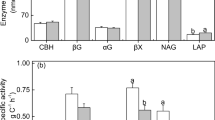
Abstract
According to the resource allocation model for extracellular enzyme synthesis, microorganisms should preferentially allocate their resources to phosphorus (P)-acquiring enzyme synthesis when P availability is low in soils. However, the validity of this model across different soil types and soils differing in their microbial community composition has not been well demonstrated. Here we investigated whether the resource allocation model for phosphatase synthesis is applicable across different soil types (Andosols, Acrisols, Cambisols, and Fluvisols) and land uses (arable and forest), and we examined which soil test P and/or P fraction microorganisms responded to when investing their resources in phosphatase synthesis in the soils. The ratio of alkaline phosphatase (ALP) to β-d-glucosidase (BG) activities in the arable soils and the ratio of acid phosphatase (ACP) to BG activities in the forest soils were significantly negatively related with the available inorganic P concentration. We also observed significant effects of available inorganic P, pH, soil types, and land uses on the (ACP + ALP)/BG ratio when the data for the arable and forest soils were combined and used in a stepwise multiple regression analysis. These results suggest that microbial resource allocation for phosphatase synthesis is primarily controlled by available inorganic P concentration and soil pH, but the effects of soil types and land uses are also significant.



Similar content being viewed by others
References
Averill C (2014) Divergence in plant and microbial allocation strategies explains continental patterns in microbial allocation and biogeochemical fluxes. Ecol Lett 17:1202–1210
Cleveland CC, Townsend AR, Schmidt SK (2002) Phosphorus limitation of microbial processes in moist tropical forests: evidence from short-term laboratory incubations and field studies. Ecosystems 5:680–691
Condron LM, Turner BL, Cade-Menun BJ (2005) Chemistry and dynamics of soil organic phosphorus. In: Sims JT, Sharpley AN (eds) Phosphorus: agriculture and the environment. American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, Madison, pp 87–121
Crews TE (1996) The supply of phosphorus from native, inorganic phosphorus pools in continuously cultivated Mexican agroecosystems. Agric Ecosyst Environ 57:197–208
Cross AF, Schlesinger WH (1995) A literature review and evaluation of the Hedley fractionation: applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 64:197–214
Dobermann A, George T, Thevs N (2002) Phosphorus fertilizer effects on soil phosphorus pools in acid upland soils. Soil Sci Soc Am J 66:652–660
Fanin N, Moorhead D, Bertrand I (2016) Eco-enzymatic stoichiometry and enzymatic vectors reveal differential C, N, P dynamics in decaying litter along a land-use gradient. Biogeochemistry 129:21–36
FAO (2006) World reference base for soil resources 2006: A framework for international classification, correlation and communication. World soil resources reports 103. Rome
Fisher LD, van Belle G (1993) Biostatistics: a methodology for the health sciences. Wiley, New York
Hedley HJ, Stewart JWB, Chauhan BS (1982) Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci Soc Am J 46:970–976
Hill BH, Elonen CM, Jicha TM, Bolgrien DW, Moffett MF (2010) Sediment microbial enzyme activity as an indicator of nutrient limitation in the great rivers of the Upper Mississippi River basin. Biogeochemistry 97:195–209
Hoshino YT, Morimoto S, Hayatsu M, Nagaoka K, Suzuki C, Karasawa T, Takenaka M, Akiyama H (2011) Effect of soil type and fertilizer management on archaeal community in upland field soils. Microbes Environ 26:307–316
Koide T, Sakai K, Kawahara S, Usui Y, Nakahara O, Hatano R (2005) Response to nitrogen loading in a P-limited larch forest ecosystem in Sapporo, Japan. Phyton 45:443–450
Kunito T, Akagi Y, Park HD, Toda H (2009) Influences of nitrogen and phosphorus addition on polyphenol oxidase activity in a forested Andisol. Eur J For Res 128:361–366
Kunito T, Nagaoka K (2009) Effects of plant litter type and additions of nitrogen and phosphorus on bacterial community-level physiological profiles in a brown forest soil. Microbes Environ 24:68–71
Kunito T, Tobitani T, Moro H, Toda H (2012a) Phosphorus limitation in microorganisms leads to high phosphomonoesterase activity in acid forest soils. Pedobiologia 55:263–270
Kunito T, Tsunekawa M, Yoshida S, Park H, Toda H, Nagaoka K, Saeki K (2012b) Soil properties affecting phosphorus forms and phosphatase activities in Japanese forest soils: soil microorganisms may be limited by phosphorus. Soil Sci 177:39–46
Landeweert R, Hoffland E, Finlay RD, Kuyper TW, van Breemen N (2001) Linking plants to rocks: ectomycorrhizal fungi mobilize nutrients from minerals. Trends Ecol Evol 16:248–254
Liao X, Inglett PW, Inglett KS (2014) Vegetation and microbial indicators of nutrient status: testing their consistency and sufficiency in restored calcareous wetlands. Ecol Indic 46:358–366
Magid J, Tiessen H, Condron LM (1996) Dynamics of organic phosphorus in soils under natural and agricultural ecosystems. In: Piccolo A (ed) Humic substances in terrestrial ecosystems. Elsevier, Amsterdam, pp 429–466
McGill WB, Cole CV (1981) Comparative aspects of cycling of organic C, N, S and P through soil organic matter. Geoderma 26:267–286
Mishima S, Itahashi S, Kimura R, Inoue T (2003) Trends of phosphate fertilizer demand and phosphate balance in farmland soils in Japan. Soil Sci Plant Nutr 49:39–45
Miura K, Nishio T (2004) Influences of application of organic materials and soil types on nitrogen balance and nitrate nitrogen concentration of soil solution in carrot cultivation. Jpn J Soil Sci Plant Nutr 75:459–465 In Japanese
Moorhead DL, Sinsabaugh RL, Hill BH, Weintraub MN (2016) Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol Biochem 93:1–7
Moro H (2015) Evaluating the nutrient availability in soils using soil enzymes. Interdisciplinary Graduate School of Science and Technology, Shinshu University, Japan, Thesis
Moro H, Kunito T, Sato T (2015) Assessment of phosphorus bioavailability in cultivated Andisols from a long-term fertilization field experiment using chemical extractions and soil enzyme activities. Arch Agron Soil Sci 61:1107–1123
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nanzyo M (1997) Available phosphorus. In: Editorial Committee for Analytical Methods for Soil Environment (ed) Dojo-kankyo-bunsekiho: analytical methods for soil environment. Hakuyusha, Tokyo, pp 267–273 In Japanese
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Schlesinger WH, Bruijnzeel LA, Bush MB, Klein EM, Mace KA, Raikes JA, Whittaker RJ (1998) The biogeochemistry of phosphorus after the first century of soil development on Rakata Island, Krakatau, Indonesia. Biogeochemistry 40:37–55
Shoji S, Nanzyo M, Dahlgren RA (1993) Volcanic ash soils. Elsevier, Amsterdam
Sinsabaugh RL, Follstad Shah JJ (2012) Ecoenzymatic stoichiometry and ecological theory. Annu Rev Ecol Evol Syst 43:313–343
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C, Contosta AR, Cusack D, Frey S, Gallo ME, Gartner TB, Hobbie SE, Holland K, Keeler BL, Powers JS, Stursova M, Takacs-Vesbach C, Waldrop MP, Wallenstein MD, Zak DR, Zeglin LH (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264
Sinsabaugh RL, Moorhead DL (1994) Resource allocation to extracellular enzyme production: a model for nitrogen and phosphorus control of litter decomposition. Soil Biol Biochem 26:1305–1311
Sui Y, Thompson ML, Shang C (1999) Fractionation of phosphorus in a Mollisol amended with biosolids. Soil Sci Soc Am J 63:1174–1180
Suzuki C, Nagaoka K, Shimada A, Takenaka M (2009) Bacterial communities are more dependent on soil type than fertilizer type, but the reverse is true for fungal communities. Soil Sci Plant Nutr 55:80–90
Tabatabai MA (1994) Soil enzymes. In: Weaver RW, Angle S, Bottomley P, Bezdicek D, Smith S, Tabatabai A, Wollum A (eds) Methods of soil analysis, part 2, Microbiological and biochemical properties. Soil Science Society of America, Madison, pp 775–833
Tate RL III (2000) Soil Microbiology, 2nd edn. Wiley, New York
Tiessen H, Moir JO (2008) Characterization of available P by sequential extraction. In: Carter MR, Gregorich EG (eds) Soil sampling and methods of analysis, 2nd edn. CRC Press, Boca Raton, pp 293–306
Truog E (1930) The determination of the readily available phosphorus of soils. J Am Soc Agron 22:874–882
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: mechanisms implications, and nitrogen–phosphorus interactions. Ecol Appl 20:5–15
Waring BG, Weintraub SR, Sinsabaugh RL (2014) Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry 117:101–113
Xu Z, Yu G, Zhang X, He N, Wang Q, Wang S, Wang R, Zhao N, Jia Y, Wang C (2017) Soil enzyme activity and stoichiometry along the North-South Transect in eastern China (NSTEC). Soil Biol Biochem 104:152–163
Acknowledgements
This study was supported by JSPS KAKENHI Grant Number JP24510012 and JP26292035.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Sasha C. Reed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fujita, K., Kunito, T., Moro, H. et al. Microbial resource allocation for phosphatase synthesis reflects the availability of inorganic phosphorus across various soils. Biogeochemistry 136, 325–339 (2017). https://doi.org/10.1007/s10533-017-0398-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-017-0398-6