Abstract
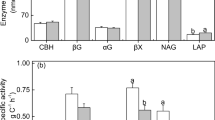
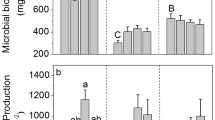
Although northern temperate forests are generally not considered phosphorus (P) limited, ecosystem P limitation may occur on highly weathered or strongly acidic soils where bioavailable inorganic P is low. In such environments, soil organisms may compensate by increasing the utilization of organic P via the production of extracellular enzymes to prevent limitation. In this study, we experimentally increased available P and/or pH in several acidic eastern deciduous forests underlain by glaciated and unglaciated soils in eastern Ohio, USA. We hypothesized that where inorganic P is low; soil microbes are able to access organic P by increasing production of phosphatase enzymes, thereby overcoming biogeochemical P limitations. We measured surface soil for: available P pools, N mineralization and nitrification rates, total C and N, enzymes responsible for C, N, and P hydrolysis, and microbial community composition (PLFA). Increasing surface soil pH a whole unit had little effect on microbial community composition, but increased N cycling rates in unglaciated soils. Phosphorus additions suppressed phosphatase activities over 60% in the unglaciated soils but were unchanged in the glaciated soils. All treatments had minimal influence on microbial biomass, but available pools of P strongly correlated with microbial composition. Microbes may be dependent on sources of organic P in some forest ecosystems and from a microbial perspective soil pH might be less important overall than P availability. Although our sampling was conducted less than 1 year after treatment initiation, microbial community composition was strongly influenced by available P pools and these effects may be greater than short-term increases in soil pH.




Similar content being viewed by others
References
Aber JD, Magill A, Boone R, Melillo JM, Steudler P, Bowden R (1993) Plant and soil responses to chronic nitrogen additions at the Harvard Forest, Massachusetts. Ecol Appl 3(1):156–166
Anderson TR, Hessen DO, Elser JJ, Urabe J (2005) Metabolic stoichiometry and the fate of excess carbon and nutrients in consumers. Am Nat 165(1):1–15
Attiwill P, Adams M (1993) Nutrient cycling in forests. New Phytol 124(4):561–582
Bååth E, Anderson TH (2003) Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol Biochem 35(7):955–963
Bramley RGV, White RE (1991) An analysis of variability in the activity of nitrifiers in a soils under pasture. Aust J Soil Res 29(1):95–108
Cleveland CC, Townsend AR, Schmidt SK (2002) Phosphorus limitation of microbial processes in moist tropical forests: evidence from short-term laboratory incubations and field studies. Ecosystems 5(7):680–691
Crews TE, Kitayama K, Fownes JH, Riley RH, Herbert DA, Muellerdombois D, Vitousek PM (1995) Changes in soil-phosphorus fractions and ecosystem dynamics across a long chronosequence in Hawaii. Ecology 76(5):1407–1424
Cross AF, Schlesinger WH (1995) A literature-review and evaluation of the Hedley fractionation-applications to the biogeochemical cycle of soil-phosphorus in natural ecosystems. Geoderma 64(3–4):197–214
Cruz AF, Hamel C, Hanson K, Selles F, Zentner RP (2009) Thirty-seven years of soil nitrogen and phosphorus fertility management shapes the structure and function of the soil microbial community in a Brown Chernozem. Plant Soil 315(1–2):173–184
DeForest JL (2009) The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and L-DOPA. Soil Biol Biochem 41(6):1180–1186
DeForest JL, Scott LG (2010) Available organic soil phosphorus has an important influence on microbial community composition. Soil Sci Soc Am J 74(6):2059–2066
DeForest JL, Zak DR, Pregitzer KS, Burton AJ (2004) Atmospheric nitrate deposition and the microbial degradation of cellobiose and vanillin in a northern hardwood forest. Soil Biol Biochem 36(6):965–971
Demetz M, Insam H (1999) Phosphorus availability in a forest soil determined with a respiratory assay compared to chemical methods. Geoderma 89(3–4):259–271
Duah-Yentumi S, Ronn R, Christensen S (1998) Nutrients limiting microbial growth in a tropical forest soil of Ghana under different management. Appl Soil Ecol 8(1–3):19–24
Ehlers K, Bakken LR, Frostegård Å, Frossard E, Bünemann EK (2010) Phosphorus limitation in a Ferralsol: impact on microbial activity and cell internal P pools. Soil Biol Biochem 42(4):558–566
Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10(12):1135–1142
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci USA 103(3):626–631
Finzi A (2009) Decades of atmospheric deposition have not resulted in widespread phosphorus limitation or saturation of tree demand for nitrogen in southern New England. Biogeochemistry 92:217–229
Fiorentino I, Fahey TJ, Groffman PM, Driscoll CT, Eagar C, Siccama TG (2003) Initial responses of phosphorus biogeochemistry to calcium addition in a northern hardwood forest ecosystem. Can J For Res-Rev Can Rech For 33(10):1864–1873
Gallardo A, Schlesinger WH (1994) Factors limiting microbial biomass in the mineral soil and forest floor of a warm-temperate forest. Soil Biol Biochem 26(10):1409–1415
Giesler R, Högberg M, Högberg P (1998) Soil chemistry and plants in Fennoscandian boreal forest as exemplified by a local gradient. Ecology 79(1):119–137
Giesler R, Petersson T, Högberg P (2002) Phosphorus limitation in boreal forests: effects of aluminum and iron accumulation in the humus layer. Ecosystems 5(3):300–314
Goldberg S, Davis JA, Hem JD (1996) The surface chemistry of aluminum oxides and hydroxides. In: Sposito G (ed) The environmental chemistry of aluminum, 2nd edn. CRC Lewis Publishers, Boca Raton, FL, pp 271–332
Gress SE, Nichols TD, Northcraft CC, Peterjohn WT (2007) Nutrient limitation in soils exhibiting differing nitrogen availabilities: What lies beyond nitrogen saturation? Ecology 88(1):119–130
Groffman PM, Fisk MC, Driscoll CT, Likens GE, Fahey TJ, Eagar C, Pardo LH (2006) Calcium additions and microbial nitrogen cycle processes in a northern hardwood forest. Ecosystems 9(8):1289–1305
Guckert J, Antworth C, Nichols P, White D (1985) Phospholipid, ester-linked fatty acid profiles as reproducible assays for changes in prokaryotic community structure of estuarine sediments. FEMS Microb Ecol 31:147–158
Johnson AH, Frizano J, Vann DR (2003) Biogeochemical implications of labile phosphorus in forest soils determined by the Hedley fractionation procedure. Oecologia 135(4):487–499
Kempers AJ, Zweers A (1986) Ammonium determination in soil extracts by the salicylate method. Commun Soil Sci Plant Anal 17(7):715–723
Krashevska V, Maraun M, Ruess L, Scheu S (2010) Carbon and nutrient limitation of soil microorganisms and microbial grazers in a tropical montane rain forest. Oikos 119(6):1020–1028
Kuo S (1996) Phosphorus. In: Sparks DL (ed) Methods of soil analysis. Part 3. Chemical methods, vol 5. ASA and SSSA, Madison, WI, pp 869–919
Lagerström A, Esberg C, Wardle DA, Giesler R (2009) Soil phosphorus and microbial response to a long-term wildfire chronosequence in northern Sweden. Biogeochemistry 95(2–3):199–213
Lajtha K, Driscoll C, Jarrell W, Elliott E (1999) Soil phosphorus: characterization and total elemental analysis. In: Robertson G, Coleman D, Bledsoe C, Sollins P (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York, pp 124–127
Liptzin D, Silver WL (2009) Effects of carbon additions on iron reduction and phosphorus availability in a humid tropical forest soil. Soil Biol Biochem 41(8):1696–1702
McCune B, Grace J (2002) Analysis of ecological communities. MJM Software Design, Gleneden Beach, OR
McGill WB, Cole CV (1981) Comparative aspects of cycling of organic C, N, S and P through soil organic-matter. Geoderma 26(4):267–286
Minick KJ, Fisk MC, Groffman PM (2011) Calcium and phosphorus interact to reduce mid-growing season net nitrogen mineralization potential in organic horizons in a northern hardwood forest. Soil Biol Biochem 43(2):271–279
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide-Biol Chem 5(1):62–71
Mitchell CC, Delaney DP, Balkcom KS (2008) A historical summary of Alabama’s Old Rotation (circa 1896): the world’s oldest, continuous cotton experiment. Agron J 100(5):1493–1498
Olander LP, Vitousek PM (2000) Regulation of soil phosphatase and chitinase activity by N and P availability. Biogeochemistry 49(2):175–190
Peng YY, Thomas SC (2010) Influence of non-nitrogenous soil amendments on soil CO2 efflux and fine root production in an N-saturated northern hardwood forest. Ecosystems 13(8):1145–1156
Phoenix GK, Booth RE, Leake JR, Read DJ, Grime JP, Lee JA (2004) Simulated pollutant nitrogen deposition increases P demand and enhances root-surface phosphatase activities of three plant functional types in a calcareous grassland. New Phytol 161(1):279–289
Porder S, Vitousek PM, Chadwick OA, Chamberlain CP, Hilley GE (2007) Uplift, erosion, and phosphorus limitation in terrestrial ecosystems. Ecosystems 10(1):158–170
R-Project (2010) R: a language and environment for statistical computing. v.2.12.0. The R Foundation for Statistical Computing, Vienna. URL:http://www.R-project.org
Robertson G, Groffman P (2007) Nitrogen transformations. In: EA P (ed) Soil microbiology, ecology, and biochemistry, vol 3. Academic Press, Ney York, NY, pp 341–364
Robertson G, Sollins P, Ellis B, Lajtha K (1999) Exchangeable ions, pH, and cation exchange capacity. In: Robertson G (ed) Standard soil methods for long-term ecological research. Oxford University Press, New York
Rojo M, Carcedo S, Mateos M (1990) Distribution and characterization of phosphatase and organic phosphorus in soil fractions. Soil Biol Biochem 22:169–174
Rousk J, Brookes PC, Bååth E (2010) The microbial PLFA composition as affected by pH in an arable soil. Soil Biol Biochem 42(3):516–520
Sims JT (1996) Lime requirement. In: Sparks DL (ed) Methods of soil analysis. Part 3. Chemical methods, vol 5. ASA and SSSA, Madison, WI, pp 491–515
Tanner EVJ (1981) The decomposition of leaf litter in Jamaican montane rain forests. J Ecol 69(1):263–275
Thomas G, Hargrove W (1984) The chemistry of soil acidity. In: Adams F (ed) Soil acidity and liming. American Society of Agronomy, Madison, WI
Tiessen H, Frossard E, Mermut AR, Nyamekye AL (1991) Phosphorus sorption and properties of ferruginous nodules from semiarid soils from Ghana and Brazil. Geoderma 48(3–4):373–389
Townsend AR, Asner GP, Cleveland CC, Lefer ME, Bustamante MMC (2002) Unexpected changes in soil phosphorus dynamics along pasture chronosequences in the humid tropics. J Geophys Res-Atmos 107(D20):9
Turner BL (2005) Storage-induced changes in phosphorus solubility of air-dried soils. Soil Sci Soc Am J 69(3):630–633
Turner BL (2008) Resource partitioning for soil phosphorus: a hypothesis. J Ecol 96(4):698–702
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157(3):423–447
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea—how can it occur. Biogeochemistry 13(2):87–115
Vitousek PM, Sanford RL (1986) Nutrient cycling in moist tropical forests. Annu Rev Ecol Syst 17:137–167
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecol Appl 20(1):5–15
Walker L, Syers J (1976) The fate of phosphorus during pedogenesis. Geoderma 15:1–19
Weand MP, Arthur MA, Lovett GM, Sikora F, Weathers KC (2010) The phosphorus status of northern hardwoods differs by species but is unaffected by nitrogen fertilization. Biogeochemistry 97(2–3):159–181
White D, Davis W, Nickels J, King J, Bobbie R (1979) Determination of the sedimentary microbial biomass by extractible lipid phosphate. Oecologia 40:51–62
Acknowledgments
This research was supported by a grant from the National Science Foundation (DEB 0918681 and DEB 0918167). We thank Charlotte Hewins, our undergraduate research assistants Alanna Shaw and Natalie Romito, and our high school intern, Adealiah Bennett, for help with field sampling and/or laboratory analysis. We also thank Charlotte Hewins, Gary Tobaj, Matthew Lurch, Keith Gilland, Lindsay Scott, Ryan Homsher, Clinton Calhoun, and Scott Fisher for help with establishing our treatments. Special thanks to Maria Farinacci for spreading nearly seven tons of lime herself. We thank Douglas Sturtz of USDA-ARS (University of Toledo) for running samples on the ICP-MS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
DeForest, J.L., Smemo, K.A., Burke, D.J. et al. Soil microbial responses to elevated phosphorus and pH in acidic temperate deciduous forests. Biogeochemistry 109, 189–202 (2012). https://doi.org/10.1007/s10533-011-9619-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-011-9619-6