Abstract
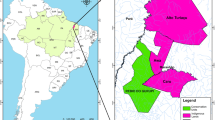
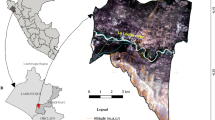
Understanding the nature and extent of ecosystem boundaries has important implications for the management and conservation of biodiversity. However, characterizing and establishing such boundary limits has been a persistent challenge worldwide. The Cerrado–Amazonia transition (CAT) in Brazil is the world’s largest savanna-forest transition. However, the CAT is represented in official maps used by Brazilian governmental agencies as a simple line separating the two biomes. Here, we demonstrate that the CAT is in fact broad, complex and interdigitating and that its traditional linear representation is not adequate for recognizing and conserving biodiversity in this region. Over the 30 years of our analysis, the CAT suffered more deforestation than the forests and savannas in each individual biomes (Amazonia and Cerrado). The complexity of tropical savanna-forest boundaries has been misunderstood and misrepresented by current maps, severely threatening the complex CAT biota. As a consequence, vegetation losses have reached levels close to collapse in areas of intense human activity.





Similar content being viewed by others
References
Ab’Sáber AN (1958) Conhecimento sobre as flutuações climáticas do quaternário no Brasil. Notícia Geomorfológica 01:24–30
Ab’Saber AN (2002) Bases para o estudo dos ecossistemas da Amazônia brasileira. Estudos Avançados 16:7–30
Adams JB, Sabol DE, Kapos V, Almeida Filho R, Roberts DA, Smith MO, Gillespie AR (1995) Classification of multispectral images based on fractions of endmembers: application to land-cover change in the Brazilian Amazon. Remote Sens Environ 52:137–154
Alencar A, Nepstad D, Mcgrath D, Moutinho P, Pacheco P, Vera Diaz MDC, Soares-Filho B (2004) Desmatamento na Amazônia: indo além da ‘‘emergência crônica’’. IPAM, Belém
Alvares CA, Stape JL, Sentelhas PC, Goncalves JLD, Sparovek G (2013) Köppen’s climate classification map for Brazil. Meteorol Z 22:711–728
Behling H, Hooghiemstra H (2000) Holocene Amazon rainforest–savanna dynamics and climatic implications: high-resolution pollen record from Laguna Loma Linda in eastern Colombia. J Quat Sci 15:687–695
Brando PM, Coe MT, Defries R, Azevedo AA (2013) Ecology, economy and management of an agroindustrial frontier landscape in the southeast Amazon. Phil Trans R Soc B 368:1–9
Brasil (1981) Ministério das Minas e Energia. Secretaria Geral. Projeto RADAMBRASIL. Folha SD.22 Goiás: geologia, geomorfologia, pedologia, vegetação, uso potencial da terra. Rio de Janeiro, v.25 636p
Breukelen MR, Vonhof HB, Hellstrom JC, Wester WCG, Kroon D (2008) Fossil dripwater in stalagmites reveals Holocene temperature and rainfall variation in Amazonia. Earth Planet Sci Lett 275:54–60
Congalton RG (1997) Exploring and evaluating the consequences of vector-to-raster and raster-to-vector conversion. Photogramm Eng Remote Sens 63:425–434
da Silva A, Mews H, Marimon-Junior BH, de Oliveira B, Morandi P, Oliveras I, Marimon BS (2018) Recurrent wildfires drive rapid taxonomic homogenization of seasonally flooded Neotropical forests. Environ Conserv 45(4):378–386
Davidson EA, de Araújo AC, Artaxo P, Balch JK, Brown IF, Bustamante MM, Coe MT, DeFries RS, Keller M, Longo M, Munger JW, Schroeder W, Soares-Filho BS, Sousa CM Jr, Wofsy SC (2012) The Amazon basin in transition. Nature 481:321–328
de Oliveira B, Junior BH, Mews HA, Valadão MB, Marimon BS (2017) Unraveling the ecosystem functions in the Amazonia-Cerrado transition: evidence of hyperdynamic nutrient cycling. Plant Ecol 218(2):225–239
Ducke A, Black GA (1953) Phytogeographical notes on the Brazilian Amazon. Academia Brazileira de Ciencias
EMBRAPA (2013) Código Florestal - adequação ambiental da paisagem rural: área de Reserva Legal. Available in: https://www.embrapa.br/codigo-florestal/area-de-reserva-legal-arl. Accessed 14 Nov 2018
ENVI (2004) ENVI Version 4.1 User’s Guide, Research Systems, Inc
ERDAS (2011) ERDAS Imagine Spectral Analysis Users Guide. ERDAS Imagine
Esquivel‐Muelbert A, Baker TR, Dexter KG, Lewis SL, Brienen RJW, Feldpausch TR, Lloyd J, Monteagudo‐Mendoza A, Arroyo L et al (2018) Compositional response of Amazon forests to climate change. Glob Change Biol. https://doi.org/10.1111/gcb.14413
ESRI (2011) Using ArcGIS Desktop: Realease 10. Instituto de Pesquisas Ambientais, Redlands
Fearnside PM (2005) Desmatamento na Amazônia brasileira: história, índices e conseqüências. Megadiversidade 1:113–123
Gloor M, Brienen RJW, Galbraith D, Feldpausch TR, Schöngart J, Guyot JL, Phillips OL (2013) Intensification of the Amazon hydrological cycle over the last two decades. Geophys Res Lett 40:1729–1733
Hoffmann WA, Jackson RB (2000) Vegetation-climate feedbacks in the conversion of tropical savanna to grassland. J Clim 13:1593–1602
IBGE (2004) Instituto Brasileiro de Geografia e Estatística - Mapa de Biomas do Brasil: Primeira aproximação. Rio de Janeiro: IBGE. Escala 1:5.000.000. <ftp://geoftp.ibge.gov.br/mapas/tematicos/mapas_murais/biomas.pdf>. Accessed 13 Oct 2014
IBGE (2014) Instituto Brasileiro de Geografia e Estatística – Área territorial brasileira. http://www.ibge.gov.br/. Accessed 13 Oct 2014
Ivanauskas NM, Monteiro R, Rodrigues RR (2008) Classificação fitogeográficas das florestas do Alto Rio Xingu. Acta Amazonica 38:387–402
Levis C, Costa FR, Bongers F, Peña-Claros M, Clement CR, Junqueira AB, Neves EG, Tamanaha EK, Figueiredo FO, Salomão RP, Castilho CV (2017) Persistent effects of pre-Columbian plant domestication on Amazonian forest composition. Science 355(6328):925–931
Lewis SL, Brando PM, Phillips OL, van der Heijden GM, Nepstad D (2011) The 2010 amazon drought. Science 331:554
Marengo JA, Tomasella J, Alves LM, Soares WR, Rodriguez DA (2011) The drought of 2010 in the context of historical droughts in the Amazon region. Geophys Res Lett 38:1–5
Marimon BS, Felfili JM, Haridasan M (2001) Studies in monodominant forests in eastern Mato Grosso, Brazil: I. A forest of Brosimum rubescens Taub. Edinb J Bot 58:123–137
Marimon BS, Lima ES, Duarte TG, Chieregatto LC, Ratter JA (2006) Observations on the vegetation of northeastern Mato Grosso, Brazil. IV. An analysis of the Cerrado-Amazonian Forest ecotone. Edinb J Bot 63:323–341
Marimon BS, Marimon-Junior BH, Mews HA et al (2012) Floristics of floodplain ‘murundus’ of the Pantanal of Araguaia, Mato Grosso, Brazil. Acta Botanica Brasilica 26:181–196
Marimon BS, Marimon-Junior BH, Feldpausch TR, Santos CO, Mews HA, Lopez-Gonzalez G, Lloyd J, Franczak DD, Oliveira EA, Maracahipes L, Miguel A, Lenza E, Phillips OL (2014) Disequilibrium and hyperdynamic tree turnover at the forest–cerrado transition zone in southern Amazonia. Plant Ecol Divers 7:37–41
Marimon-Junior BH, Haridasan M (2005) Comparação da vegetação arbórea e características edáficas de um cerradão e um cerrado sensu stricto em áreas adjacentes sobre solo distrófico no leste de Mato Grosso, Brasil. Acta Bot Bras 19:913–992
Matricardi EAT, Skole DL, Pedlowski MA, Chomentowski K, Fernandes LC (2010) Assessment of tropical forest degradation by selective logging and fire using Landsat imagery. Remote Sens Environ 114:1117–1129
Matricardi EAT, Skole DL, Pedlowski MA, Chomentowski K (2013) Assessment of forest disturbances by selective logging and forest fires in the Brazilian Amazon using Landsat data. Int J Remote Sens 34:1057–1086
Meneses PR, Almeida T (2012) Introdução ao Processamento de Imagens de Sensoriamento Remoto. UnB/CNPq, Brasília
Metzger JP (2006) Como lidar com regras pouco óbvias para conservação da biodiversidade em paisagens fragmentadas. Natureza & Conservação 4:11–23
Mews HA, Marimon BS, Ratter JA (2012) Observations on the vegetation of Mato Grosso, Brazil. V.* changes in the woody species diversity of a forest in the Cerrado-Amazonian Forest Transition zone and notes on the forests of the region. Edinb J Bot 69:239–253
Mittermeier RA, Myers N, Thomsen JB, Fonseca GAB, Olivieri S (1998) Biodiversity hotspots and major tropical wilderness areas: approaches to setting conservation priorities. Conserv Biol 12:516–520
Morandi PS, Marimon-Junior BH, Oliveira E, Reis S, Valadão MBX, Forsthofer M, Marimon BS (2016a) Vegetation Succesion in the Cerrado-Amazonian forest transition zone of Mato Grosso State, Brazil. Edinb J Bot 73:83–93
Morandi PS, Marimon BS, Eisenlohr PV, Marimon-Junior BH, Oliveira-Santos C, Feldpausch TR, Oliveira EA, Reis SM, Lloyd J, Phillips OL (2016b) Patterns of tree species composition at watershed-scale in the Amazon ‘arc of deforestation’: implications for conservation. Environ Conserv 43(4):317–326
Morandi PS, Marimon BS, Marimon-Junior BH, Ratter JA, Feldpausch TR, Colli GR, Munhoz CB, Silva-Júnior MC, Souza-Lima E, Haidar RF, Arroyo L, Araujo-Murakami A, Góis FA, Walter BMT, Ribeiro JS, Françoso R, Elias F, Oliveira EA, Reis SM, Oliveira B, Neves EC, Nogueira DS, Lima HS, Carvalho TP, Rodrigues S, Villarroel D, Felfili JM, Phillips OL (2018) Tree diversity and above-ground biomass in the South America Cerrado biome and their conservation implications. Biodivers Conserv. https://doi.org/10.1007/s10531-018-1589-8
Nogueira EM, Fearnside PM, Nelson BW, França MB (2007) Wood density in forests of Brazil’s ‘arc of deforestation’: implications for biomass and flux of carbon from land-use change in Amazonia. For Ecol Manage 248:119–135
Nogueira EM, Nelson BW, Fearnside PM, França MB, Oliveira ACA (2008) Tree height in Brazil’s ‘arc of deforestation’: shorter trees in south and southwest Amazonia imply lower biomass. For Ecol Manage 255:2963–2972
Oliveira B, Marimon-Junior BH, Mews HA, Valadão MBX, Marimon BS (2017) Unraveling the ecosystem functions in the Amazonia-Cerrado transition: evidence of hyperdynamic nutrient cycling. Plant Ecol 218:225–239
Overbeck GE, Vélez-Martin E, Scarano FR et al (2015) Conservation in Brazil needs to include non-forest ecosystems. Divers Distrib 21:1455–1460
Pacheco CSGR, Oliveira NMGA (2016) Conservação das espécies vegetais em paleoambientes dunares na APA Dunas e Veredas do Baixo-Médio São Francisco, Bahia, Brasil. Nat Resour 6:6–17
Papp L (2012) Comentários ao Novo Código Florestal Brasileiro - Lei 12.651/12. 1a. Ed. Millennium, 352 p
Passos FB, Marimon BS, Phillips OL, Morandi PS, Neves EC, Elias S, Reis SM, Oiveira B, Feldpausch TR, Marimon-Junior BH (2018) Savanna turning into forest: concerted vegetation change at the ecotone between the Amazon and “Cerrado” biomes. Braz J Bot 41:611–619
Peixoto KS, Marimon-Junior BH, Marimon BS, Elias F, de Farias J, Freitag R, Mews HA, das Neves EC, Prestes NC, Malhi Y (2017) Unravelling ecosystem functions at the Amazonia-Cerrado transition: II. Carbon stocks and CO2 soil efflux in cerradao forest undergoing ecological succession. Acta Oecologica 82:23–31
Peixoto KD, Marimon-Junior BH, Cavalheiro KA, Silva NA, das Neves EC, Freitag R, Mews HA, Valadão MB (2018) Assessing the effects of rainfall reduction on litterfall and the litter layer in phytophysiognomies of the Amazonia-Cerrado transition. Braz J Bot 41(3):589–600
Pessenda LC, Boulet R, Aravena R, Rosolen V, Gouveia SEM, Ribeiro AS, Lamotte M (2001) Origin and dynamics of soil organic matter and vegetation changes during the Holocene in a forest-savanna transition zone, Brazilian Amazon region. Holocene 11:250–254
Pessenda LCR, Gouveia SEM, de Souza Ribeiro A, De Oliveira PE, Aravena R (2010) Late Pleistocene and Holocene vegetation changes in northeastern Brazil determined from carbon isotopes and charcoal records in soils. Palaeogeogr Palaeoclimatol Palaeoecol 297:597–608
Phillips OL, Malhi Y, Higuchi N et al (1998) Changes in the carbon balance of tropical forests: evidence from long-term plots. Science 282(5388):439–442
Ratter JA (1993) Transition between cerrado and forest vegetation in Brazil. In: Furley PA, Proctor J, Ratter JA (eds) Nature and dynamics of forest-Savanna Boundaries. Chapman & hall, London, pp 417–429
Ratter JA, Richards PW, Argent G, Gifford DR (1973) Observations on the vegetation of northeastern Mato Grosso – The wood vegetation types of the Xavantina – Cachimbo Expedition Area. Philos Trans R Soc 226:229–492
Ratter JA, Ribeiro JF, Bridgewater S (1997) The Brazilian Cerrado vegetation and threats to its biodiversity. Ann Bot 80:223–230
Ratter JA, Bridgewater S, Ribeiro JF (2003) Analysis of the floristic composition of the Brazilian cerrado vegetation III: comparison of the woody vegetation of 376 areas. Edinb J Bot 60:57–109
Ribeiro JF, Walter BMT (2008) As principais fitofisionomias do bioma Cerrado. In: Sano SM, Almeida SP (eds) Cerrado: Ecologia e flora, 2nd edn. Embrapa–Cerrados, Brasilia, pp 151–212
Ronnenberg KL (2013) How landscapes change: human disturbance and ecosystem fragmentation in the Americas. Springer, New York
Silvério DV, Brando PM, Balch JK, Putz FE, Nepstad DC, Oliveira-Santos C, Bustamante MMC (2013) Testing the Amazon savannization hypothesis: fire effects on invasion of a neotropical forest by native cerrado and exotic pasture grasses. Phil Trans R Soc B 368:1–9
Soares LC (1953) Limites meridionais e orientais da área de ocorrência da Floresta amazônica em território Brasileiro. Rev bras Geografia 1:3–122
Torello-Raventos M, Feldpausch TR, Veenendaal E, Schrodt F, Saiz G, Domingues TF, Marimon-Junior BH (2013) On the delineation of tropical vegetation types with an emphasis on forest/savanna transitions. Plant Ecol Divers 6(1):101–137
Valadão MBX, Marimon JB, Oliveira BD, Lucio NW, Souza M, Marimon BS (2016) Biomass hyperdynamics as a key modulator of forest self-maintenance in a dystrophic soil in the Amazonia-Cerrado transition. Scientia Forestalis 44:475–485
Werneck FP, Nogueira C, Colli GR, Sites JW, Costa GC (2012) Climatic stability in the Brazilian Cerrado: implications for biogeographical connections of South American savannas, species richness and conservation in a biodiversity hotspot. J Biogeogr 39:1695–1706
Acknowledgements
We thank CAPES for the MSc scholarship to the first author and the National Council of Science and Technology of Brazil (CNPq) for the financial funding of the project PPBIO 457602/2012-0 (Rede Biota do Cerrado) and productivity grants to BH Marimon, BS Marimon and GR Colli. GRC thanks Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional do Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Apoio à Pesquisa do Distrito Federal (FAPDF), and the Partnerships for Enhanced Engagement in Research (PEER) program for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Guarino Rinaldi Colli.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10531_2019_1720_MOESM1_ESM.tif
Supplementary material 1 (TIFF 774 kb). Appendix Fig. A1 Filtered using a 5 km2 grid size for the reclassification and smoothing tools used as the base of the manually refined ecotone complex
10531_2019_1720_MOESM2_ESM.tif
Supplementary material 2 (TIFF 801 kb). Appendix Fig. A2 Result of the smoothing used as the base for the manually refined ecotone complex
10531_2019_1720_MOESM3_ESM.tif
Supplementary material 3 (TIFF 6593 kb). Appendix Fig. A3 Cerrado–Amazonia limit defined by considering the types of vegetation occurring in this region. The dividing line between biomes defined in this study (in blue) clearly does not match the line previously defined by the IBGE’s official mapping (in black)
10531_2019_1720_MOESM4_ESM.tif
Supplementary material 4 (TIFF 1748 kb). Appendix Fig. A4 Scheme showing events that may potentially lead to encroachment or retraction of forests or savannas, contributing to the floristic complexity of the Cerrado–Amazonia transition in Brazil
10531_2019_1720_MOESM5_ESM.tif
Supplementary material 5 (TIFF 1811 kb). Appendix Fig. A5 Land cover classes and protected areas. Indigenous Lands and Conservation Units, accounting for 16.7% of the landscape in the Cerrado–Amazonia transition in Brazil in 2014
Rights and permissions
About this article
Cite this article
Marques, E.Q., Marimon-Junior, B.H., Marimon, B.S. et al. Redefining the Cerrado–Amazonia transition: implications for conservation. Biodivers Conserv 29, 1501–1517 (2020). https://doi.org/10.1007/s10531-019-01720-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-019-01720-z