Abstract
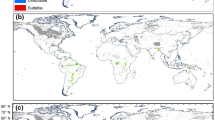
Inland aquatic ecosystems are vulnerable to both climate change and biological invasion at broad spatial scales. The aim of this study was to establish the current and future potential distribution of three invasive plant taxa, Egeria densa, Myriophyllum aquaticum and Ludwigia spp., in their native and exotic ranges. We used species distribution models (SDMs), with nine different algorithms and three global circulation models, and we restricted the suitability maps to cells containing aquatic ecosystems. The current bioclimatic range of the taxa was predicted to represent 6.6–12.3% of their suitable habitats at global scale, with a lot of variations between continents. In Europe and North America, their invasive ranges are predicted to increase up to two fold by 2070 with the highest gas emission scenario. Suitable new areas will mainly be located to the north of their current range. In other continents where they are exotic and in their native range (South America), the surface areas of suitable locations are predicted to decrease with climate change, especially for Ludwigia spp. in South America (down to −55% by 2070 with RCP 8.5 scenario). This study allows to identify areas vulnerable to ongoing invasions by aquatic plant species and thus could help the prioritisation of monitoring and management, as well as contribute to the public awareness regarding biological invasions.



Similar content being viewed by others
References
Alahuhta J, Heino J, Luoto M (2011) Climate change and the future distributions of aquatic macrophytes across boreal catchments. J Biogeogr 38:383–393. doi:10.1111/j.1365-2699.2010.02412.x
Araújo MB, New M (2007) Ensemble forecasting of species distributions. Trends Ecol Evol 22:42–47. doi:10.1016/j.tree.2006.09.010
Barbet-Massin M, Jiguet F, Albert CH, Thuiller W (2012) Selecting pseudo-absences for species distribution models: how, where and how many? Methods Ecol Evol 3:327–338. doi:10.1111/j.2041-210X.2011.00172.x
Bellard C, Thuiller W, Leroy B et al (2013) Will climate change promote future invasions? Glob Chang Biol 19:3740–3748. doi:10.1111/gcb.12344
Bellard C, Leroy B, Thuiller W et al (2016) Major drivers of invasion risks throughout the world. Ecosphere 7:1–14. doi:10.1002/ecs2.1241
Bornette G, Puijalon S (2010) Response of aquatic plants to abiotic factors: a review. Aquat Sci 73:1–14. doi:10.1007/s00027-010-0162-7
Bradshaw CJA, Leroy B, Bellard C et al (2016) Massive yet grossly underestimated costs of invasive insects. Nat Commun. doi:10.1038/ncomms12986
Breiman L (2001) Random forests. Mach Learn 45:5–32. doi:10.1023/A:1010933404324
Breiman L, Friedman JH, Olshean RA, Stone CJ (1984) Classification and regression trees. Chapman and Hall, London
Broennimann O, Guisan A (2008) Predicting current and future biological invasions: both native and invaded ranges matter. Biol Lett 4:585–589. doi:10.1098/rsbl.2008.0254
Broennimann O, Treier UA, Müller-Schärer H et al (2007) Evidence of climatic niche shift during biological invasion. Ecol Lett 10:701–709. doi:10.1111/j.1461-0248.2007.01060.x
Buisson L, Thuiller W, Casajus N et al (2010) Uncertainty in ensemble forecasting of species distribution. Glob Chang Biol 16:1145–1157. doi:10.1111/j.1365-2486.2009.02000.x
Carey MP, Sethi SA, Larsen SJ, Rich CF (2016) A primer on potential impacts, management priorities, and future directions for Elodea spp. in high latitude systems: learning from the Alaskan experience. Hydrobiologia 777:1–19. doi:10.1007/s10750-016-2767-x
Collins M, Tett SFB, Cooper C (2001) The internal climate variability of HadCM3, a version of the Hadley Centre coupled model without flux adjustments. Clim Dyn 17:61–81. doi:10.1007/s003820000094
Dandelot S, Verlaque R, Dutartre A, Cazaubon A (2005) Ecological, dynamic and taxonomic problems due to Ludwigia (Onagraceae) in France. Hydrobiologia 551:131–136. doi:10.1007/s10750-005-4455-0
Dudgeon D, Arthington AH, Gessner MO et al (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev 81:163–182. doi:10.1017/S1464793105006950
Ebeling SK, Welk E, Auge H, Bruelheide H (2008) Predicting the spread of an invasive plant: combining experiments and ecological niche model. Ecography (Cop) 31:709–719. doi:10.1111/j.1600-0587.2008.05470.x
Elith J, Ferrier S, Huettmann F, Leathwick J (2005) The evaluation strip: a new and robust method for plotting predicted responses from species distribution models. Ecol Model 186:280–289. doi:10.1016/j.ecolmodel.2004.12.007
Feijoó C, García ME, Momo F, Toja J (2002) Nutrient absorption by the submerged macrophyte Egeria densa Planch.: effect of ammonium and phosphorus availability in the water colum on growth and nutrient uptake. Limnetica 21:93–104
Finch JM, Samways MJ, Hill TR et al (2006) Application of predictive distribution modelling to invertebrates: Odonata in South Africa. Biodivers Conserv 15:4239–4251. doi:10.1007/s10531-005-3577-z
Friedman JH (1991) Multivariate adaptive regression splines. Ann Stat 19:1–67
Gallardo B, Aldridge DC (2013) The “dirty dozen”: socio-economic factors amplify the invasion potential of 12 high-risk aquatic invasive species in Great Britain and Ireland. J Appl Ecol 50:757–766. doi:10.1111/1365-2664.12079
Gallien L, Douzet R, Pratte S et al (2012) Invasive species distribution models—how violating the equilibrium. Glob Ecol Biogeogr 21:1126–1136. doi:10.1111/j.1466-8238.2012.00768.x
Gent PR, Danabasoglu G, Donner LJ et al (2011) The community climate system model version 4. J Clim 24:4973–4991. doi:10.1175/2011JCLI4083.1
Getsinger KD, Dillon CR (1984) Quiescence, growth and senescence of Egeria densa in Lake Marion. Aquat Bot 20:329–338. doi:10.1016/0304-3770(84)90096-2
Guisan A, Thuiller W (2005) Predicting species distribution: offering more than simple habitat models. Ecol Lett 8:993–1009. doi:10.1111/j.1461-0248.2005.00792.x
Guisan A, Tingley R, Baumgartner JB et al (2013) Predicting species distributions for conservation decisions. Ecol Lett 16:1424–1435. doi:10.1111/ele.12189
Hastie T, Tibshirani R (1990) Generalized additive models. Chapman and Hall, London
Hastie T, Tibshirani R, Buja A (1994) Flexible discriminant analysis by optimal scoring. J Am Stat Assoc 89:1255–1270. doi:10.2307/2290989
Heikkinen R, Leikola N, Fronzek S et al (2009) Predicting distribution patterns and recent northward range shift of an invasive aquatic plant: Elodea canadensis in Europe. BioRisk 2:1–32. doi:10.3897/biorisk.2.4
Hijmans RJ, Cameron SE, Parra JL et al (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978. doi:10.1002/joc.1276
Hussner A (2010) Growth response and root system development of the invasive Ludwigia grandiflora and Ludwigia peploides to nutrient availability and water level. Fundam Appl Limnol/Arch Hydrobiol 177:189–196. doi:10.1127/1863-9135/2010/0177-0189
Hussner A (2012) Alien aquatic plant species in European countries. Weed Res 52:297–306. doi:10.1111/j.1365-3180.2012.00926.x
Hussner A, Champion PD (2011) Myriophyllum aquaticum (Vell.) Verdcourt (parrot feather). In: Francis RA (ed) A Handbook of global freshwater invasive species. Routledge, New York, p 456
Hussner A, Meyer C, Busch J (2009) The influence of water level and nutrient availability on growth and root system development of Myriophyllum aquaticum. Weed Res 49:73–80
IPCC (2012) Managing the risks of extreme events and disasters to advance climate change adaptation. Cambridge University Press, Cambridge
Jaccard P (1901) Distribution de la flore alpine dans le Bassin des Drouces et dans quelques regions voisines. Bull la Société Vaudoise des Sci Nat 37:241–272
Kelly R, Leach K, Cameron A et al (2014) Combining global climate and regional landscape models to improve prediction of invasion risk. Divers Distrib 20:884–894. doi:10.1111/ddi.12194
Kriticos DJ, Sutherst RW, Brown JR et al (2003) Climate change and the potential distribution of an invasive alien plant: Acacia nilotica ssp. indica in Australia. J Appl Ecol 40:111–124
Lehner B, Döll P (2004) Development and validation of a global database of lakes, reservoirs and wetlands. J Hydrol 296:1–22. doi:10.1016/j.jhydrol.2004.03.028
Leroy B, Paschetta M, Canard A et al (2013) First assessment of effects of global change on threatened spiders: potential impacts on Dolomedes plantarius (Clerck) and its conservation plans. Biol Conserv 161:155–163. doi:10.1016/j.biocon.2013.03.022
Li W (2014) Environmental opportunities and constraints in the reproduction and dispersal of aquatic plants. Aquat Bot 118:62–70. doi:10.1016/j.aquabot.2014.07.008
Li W, Guo Q (2013) How to assess the prediction accuracy of species presence–absence models without absence data? Ecography (Cop) 36:788–799. doi:10.1111/j.1600-0587.2013.07585.x
Liu C, Berry PM, Dawson TP, Pearson RG (2005) Selecting thresholds of occurrence in the prediction of species distributions. Ecography (Cop) 28:385–393. doi:10.1111/j.0906-7590.2005.03957.x
Lowe S, Browne M, Boudjelas S, De Poorter M (2004) 100 of the world’s worst invasive alien species a selection from the Global Invasive Species Database. Invasive Species Specialist Group, Species Survival Commission, World Conservation Union (IUCN)
Mainka SA, Howard GW (2010) Climate change and invasive species: double jeopardy. Integr Zool 5:102–111. doi:10.1111/j.1749-4877.2010.00193.x
McCullagh P, Nelder JA (1989) Generalized linear models, 2nd edn. Chapman and Hall, London
Peterson AT, Papes M, Kluza DA (2003) Predicting the potential invasive distributions of four alien plant species in North America. Weed Sci 51:863–868
Peterson AT, Stewart A, Mohamed KI, Araújo MB (2008) Shifting global invasive potential of European plants with climate change. PLoS ONE. doi:10.1371/journal.pone.0002441
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Model 190:231–259. doi:10.1016/j.ecolmodel.2005.03.026
Qin Z, DiTommaso A, Wu RS, Huang HY (2014) Potential distribution of two Ambrosia species in China under projected climate change. Weed Res 54:520–531. doi:10.1111/wre.12100
R Development Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Ridgeway G (1999) The state of boosting. Comput Sci Stat 31:172–181
Ripley BD (1996) Neural networks and pattern recognition. Cambridge University, Cambridge
Ruaux B, Greulich S, Haury J, Berton J-P (2009) Sexual reproduction of two alien invasive Ludwigia (Onagraceae) on the middle Loire River, France. Aquat Bot 90:143–148. doi:10.1016/j.aquabot.2008.08.003
Santamaría L (2002) Why are most aquatic plants widely distributed? Dispersal, clonal growth and small-scale heterogeneity in a stressful environment. Acta Oecol 23:137–154. doi:10.1016/S1146-609X(02)01146-3
Stephens PA, Mason LR, Green RE et al (2016) Consistent response of bird populations to climate change on two continents. Science (80-) 352:84–87. doi:10.1126/science.aac4858
Stiers I, Crohain N, Josens G, Triest L (2011) Impact of three aquatic invasive species on native plants and macroinvertebrates in temperate ponds. Biol Invasions 13:2715–2726. doi:10.1007/s10530-011-9942-9
Thalmann DJK, Kikodze D, Khutsishvili M et al (2015) Areas of high conservation value in Georgia: present and future threats by invasive alien plants. Biol Invasions 17:1041–1054. doi:10.1007/s10530-014-0774-2
Thouvenot L, Haury J, Thiebaut G (2013a) A success story: water primroses, aquatic plant pests. Aquat Conserv Mar Freshw Ecosyst 23:790–803
Thouvenot L, Puech C, Martinez L et al (2013b) Strategies of the invasive macrophyte Ludwigia grandiflora in its introduced range: competition, facilitation or coexistence with native and exotic species? Aquat Bot 107:8–16. doi:10.1016/j.aquabot.2013.01.003
Thuiller W, Lafourcade B, Engler R, Araújo MB (2009) BIOMOD—a platform for ensemble forecasting of species distributions. Ecography (Cop) 32:369–373. doi:10.1111/j.1600-0587.2008.05742.x
Watts G, Battarbee RW, Bloomfield JP et al (2015) Climate change and water in the UK—past changes and future prospects. Prog Phys Geogr 39:6–28. doi:10.1177/0309133314542957
Whitehead PG, Wilby RL, Battarbee RW et al (2009) A review of the potential impacts of climate change on surface water quality. Hydrol Sci J 54:101–123. doi:10.1623/hysj.54.1.101
Yarrow M, Marin VH, Finlayson M et al (2009) The ecology of Egeria densa Planchon (Liliopsida: Alismatales): a wetland ecosystem engineer? Rev Chil Hist Nat 82:299–313
Yukimoto S, Adachi Y, Hosaka M et al (2012) A new global climate model of the Meteorological Research Institute: MRI-CGCM3. J Meteorol Soc Jpn 90A:23–64. doi:10.2151/jmsj.2012-A02
Zhang C, Boyle KJ (2010) The effect of an aquatic invasive species (Eurasian watermilfoil) on lakefront property values. Ecol Econ 70:394–404. doi:10.1016/j.ecolecon.2010.09.011
Acknowledgements
We kindly thank Márcio José Silveira for providing the occurrences of the studied taxa in Brazil, and Aldyth Nys for the English editing of the manuscript. This work was supported by a Ph.D. fellowship from the French Ministry for Higher Education and Research to MG. We would like to warmly thank the two reviewers who evaluated and contributed to improve a previous version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gillard, M., Thiébaut, G., Deleu, C. et al. Present and future distribution of three aquatic plants taxa across the world: decrease in native and increase in invasive ranges. Biol Invasions 19, 2159–2170 (2017). https://doi.org/10.1007/s10530-017-1428-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-017-1428-y