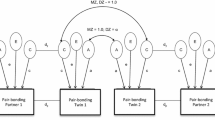
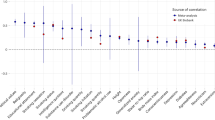
Abstract
Choice of romantic partner is an enormously important component of human life, impacting almost every facet of day-to-day existence, however; the processes underlying this choice are remarkably complex and have so far been largely resistant to scientific explanation. One consistent finding is that, on average, members of romantic dyads tend to be more alike than would be expected by chance. Selecting for self-similarity is at least partially driven by phenotypic matching wherein couples share similar phenotypes, and preferences for a number of these traits are partly genetically influenced (e.g., education, height, social attitudes and religiosity). This suggests that genetically influenced preferences for self-similarity might contribute to phenotypic matching (and thus assortative mating), but it has never been studied in actual couples. In the present study, we use a large sample of twins to model sources of variation in self-similarity between partners. Biometrical modelling revealed that very little of the variation in the tendency to assortatively mate across 14 traits was due to genetic effects (7 %) or the shared environment of twins (0 %).

Similar content being viewed by others
References
Agrawal A, Heath A, Grant J, Pergadia M, Statham D, Bucholz K, Madden P (2006) Assortative mating for cigarette smoking and for alcohol consumption in female Australian twins and their spouses. Behav Genet 36(4):553–566. doi:10.1007/s10519-006-9081-8
Boker SM, Neale MC, Maes HH, Wilde MJ, Spiegel M, Brick TR, Fox J (2011) OpenMx: an open source extended structural equation modelling framework: Psychometrika
Boomsma DI, Neale MC, Dolan CV (1999) A note on the power provided by sibships of sizes 2, 3, and 4 in genetic covariance odeling of a codominant QTL. Behav Genet 29(3):163–170
Boomsma DI, Saviouk V, Hottenga J-J, Distel MA, de Moor MHM, Vink JM, Willemsen G (2010) Genetic epidemiology of attention deficit hyperactivity disorder (ADHD Index) in adults (epidemiology of ADHD index). PLoS ONE 5(5):e10621. doi:10.1371/journal.pone.0010621
Bulmer MG (1971) The effect of selection on genetic variability. Am Nat 105(943):201–211. doi:10.1086/282718
Buunk AP, Park JH, Dubbs SL (2008) Parent-offspring conflict in mate preferences. Rev Gen Psychol 12(1):47–62. doi:10.1037/1089-2680.12.1.47
Caspi A, Herbener ES, Ozer DJ (1992) Shared experiences and the similarity of personalities: a longitudinal study of married couples. J Pers Soc Psychol 62(2):281–291. doi:10.1037/0022-3514.62.2.281
Cloninger CR, Przybeck TR, Svrakic DM (1991) The tridimensional personality questionnaire: U.S. normative data. Psychol Rep 69(3):1047–1057
Crow JF, Felsenstein J (1982) The effect of assortative mating on the genetic composition of a population. Soc Biol 29(1–2):22–35
Eastwick PW, Luchies LB, Finkel EJ, Hunt LL (2014) The predictive validity of ideal partner preferences: a review and meta-analysis. Psychol Bull 140(3):623–665. doi:10.1037/a0032432
Eysenck SBG, Eysenck HJ, Barrett P (1985) A revised version of the psychoticism scale. Personal Individ Differ 6(1):21–29. doi:10.1016/0191-8869(85)90026-1
Feingold A (1988) Matching for attractiveness in romantic partners and same-sex friends: a meta-analysis and theoretical critique. Psychol Bull 104(2):226–235. doi:10.1037/0033-2909.104.2.226
Freeman MF, Tukey JW (1950) Transformations related to the angular and the square root. Ann Math Stat 21(4):607–611. doi:10.1214/aoms/1177729756
Grant JD, Heath AC, Bucholz KK, Madden PAF, Agrawal A, Statham DJ, Martin NG (2007) Spousal concordance for alcohol dependence: evidence for assortative mating or spousal interaction effects? Alcohol Clin Exp Res 31(5):717–728. doi:10.1111/j.1530-0277.2007.00356.x
Hatemi PK, Hibbing JR, Medland SE, Keller MC, Alford JR, Smith KB, Eaves LJ (2010) Not by twins alone: using the extended family design to investigate genetic influence on political beliefs. Am J Polit Sci 54(3):798–814. doi:10.1111/j.1540-5907.2010.00461.x
Heath AC, Eaves LJ (1985) Resolving the effects of phenotype and social background on mate selection. Behav Genet 15(1):15–30. doi:10.1007/BF01071929
Heath AC, Cloninger CR, Martin NG (1994) Testing a model for the genetic structure of personality: a comparison of the personality systems of Cloninger and Eysenck. J Pers Soc Psychol 66(4):762. doi:10.1037/0022-3514.66.4.762
IBM Corp. (2013). IBM SPSS Statistics for Macintosh, Version 22.0. Armonk, NY: IBM Corp
Keller MC, Medland SE, Duncan LE (2010) Are extended twin family designs worth the trouble? A comparison of the bias, precision, and accuracy of parameters estimated in four twin family models. Behav Genet 40(3):377–393. doi:10.1007/s10519-009-9320-x
Keller MC, Garver-Apgar CE, Wright JC, Martin NG, Corley RP, Stallings MC, Zietsch BP (2013) The genetic correlation between height and IQ: shared genes or assortative mating? PLoS Genet. doi:10.1371/journal.pgen.1004329
Kendler KS, Myers J (2009) A developmental twin study of church attendance and alcohol and nicotine consumption: a model for analyzing the changing impact of genes and environment. Am J Psychiatry 166(10):1150–1155. doi:10.1176/appi.ajp.2009.09020182
Klohnen EC, Mendelsohn GA (1998) Partner selection for personality characteristics: a couple-centered approach. Pers Soc Psychol Bull 24(3):268–278. doi:10.1177/0146167298243004
Koenig LB, McGue M, Iacono WG (2009) Rearing environmental influences on religiousness: an investigation of adolescent adoptees. Personal Individ Differ 47(6):652–656. doi:10.1016/j.paid.2009.06.003
Krueger R, Moffitt T, Caspi A, Bleske A, Silva P (1998) Assortative mating for antisocial behavior: developmental and methodological implications. Behav Genet 28(3):173–186. doi:10.1023/A:1021419013124
Kurzban R, Weeden J (2005) HurryDate: mate preferences in action. Evolut Hum Behav 26(3):227–244. doi:10.1016/j.evolhumbehav.2004.08.012
Lande R (1977) The influence of the mating system on the maintenance of genetic variability in polygenic characters. Genetics 86(2):485–498
Li NP, Meltzer AL (2015) The validity of sex-differentiated mate preferences: reconciling the seemingly conflicting evidence. Evolut Behav Sci 9(2):89–106. doi:10.1037/ebs0000036
Li NP, Yong JC, Tov W, Sng O, Fletcher GJO, Valentine KA, Balliet D (2013) Mate preferences do predict attraction and choices in the early stages of mate selection. J Pers Soc Psychol 105(5):757–776. doi:10.1037/a0033777
Lykken DT, Tellegen A (1993) Is human mating adventitious or the result of lawful choice? A twin study of mate selection. J Pers Soc Psychol 65(1):56
Martin NG, Eaves LJ, Heath AC, Jardine R, Feingold LM, Eysenck HJ (1986) Transmission of social attitudes. Proc Natl Acad Sci USA 83(12):4364–4368. doi:10.1073/pnas.83.12.4364
Mascie-Taylor CGN (1989) Spouse similarity for IQ and personality and convergence. Behav Genet 19(2):223–227
Mascie-Taylor CGN, Vandenberg SG (1988) Assortative mating for IQ and personality due to propinquity and personal preference. Behav Genet 18(3):339–345. doi:10.1007/BF01260934
Nagoshi CT, Johnson RC, Ahern FM (1987) Phenotypic assortative mating vs. social homogamy among Japanese and Chinese parents in the Hawaii Family Study of Cognition. Behav Genet 17(5):477–485. doi:10.1007/BF01073114
Neale MC, Cardon LC (1992) Methodology for genetic studies of twins and families. Kluwer Academic Publishers, Boston
Nordsletten AE, Larsson H, Crowley JJ, Almqvist C, Lichtenstein P, Mataix-Cols D (2016) Patterns of nonrandom mating within and across 11 major psychiatric disorders. 73(4)
Penke L, Todd PM, Lenton AP, Fasolo B (2007) How self-assessments can guide human mating decisions. In: Geher G, Miller GF (eds) Mating intelligence: sex, relationships, and the mind’s reproductive system. Erlbaum, Mahwah, pp 37–75
Plomin R, DeFries JC, Roberts MK (1977) Assortative mating by unwed biological parents of adopted children. Science 196(4288):449–450. doi:10.1126/science.850790
Polderman TJC, Benyamin B, de Leeuw CA, Sullivan PF, van Bochoven A, Visscher PM, Posthuma D (2015) Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet Adv Online Publ. doi:10.1038/ng.3285
Posner SF, Baker L, Heath A, Martin NG (1996) Social contact, social attitudes, and twin similarity. Behav Genet 26(2):123–133. doi:10.1007/BF02359890
Posthuma D, Boomsmsa DI (2000) A note on the statistical power in extended twin designs. Behav Genet 30:147–158
Posthuma D, Beem AL, de Geus EJC, van Baal GCM, von Hjelmborg JB, Iachine I, Boomsma DI (2003) Theory and practice in quantitative genetics. Twin Res 6(5):361–376. doi:10.1375/136905203770326367
Price RA, Vandenberg SG (1980) Spouse similarity in American and Swedish couples. Behav Genet 10(1):59–71. doi:10.1007/BF01067319
Qvarnstrom A, Brommer JE, Gustafsson L (2006) Testing the genetics underlying the co-evolution of mate choice and ornament in the wild. Nat 441(7089):84–86. doi:http://www.nature.com/nature/journal/v441/n7089/suppinfo/nature04564_S1.html
R Core Team (2014) R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Retrieved from http://www.R-project.org/
Reynolds CA, Baker LA, Pedersen NL (1996) Models of spouse similarity: applications to fluid ability measured in twins and their spouses. Behav Genet 26(2):73–88. doi:10.1007/BF02359886
Reynolds CA, Baker LA, Pedersen NL (2000) Multivariate models of mixed assortment: phenotypic assortment and social homogamy for education and fluid ability. Behav Genet 30(6):455–476. doi:10.1023/A:1010250818089
Schwartz CR (2013) Trends and variation in assortative mating: causes and consequences. Ann Rev Sociol 39:451–470. doi:10.1146/annurev-soc-071312-145544
Verweij KJH, Burri AV, Zietsch BP (2012) Evidence for genetic variation in human mate preferences for sexually dimorphic physical traits. PLoS ONE 7(11):e49294. doi:10.1371/journal.pone.0049294
Verweij KJH, Burri AV, Zietsch BP (2014) Testing the prediction from sexual selection of a positive genetic correlation between human mate preferences and corresponding traits. Evolut Hum Behav 35(6):497–501. doi:10.1016/j.evolhumbehav.2014.06.009
Watson D, Klohnen EC, Casillas A, Nus Simms E, Haig J, Berry DS (2004) Match makers and deal breakers: analyses of assortative mating in newlywed couples. J Pers 72(5):1029–1068. doi:10.1111/j.0022-3506.2004.00289.x
Wilson SR (1973) The correlation between relatives under the multifactorial model with assortative mating. Ann Hum Genet 37(2):189–204. doi:10.1111/j.1469-1809.1973.tb01826.x
Wright S (1921) Systems of mating. III. Assortative mating based on somatic resemblance. Genetics 6(2):144–161
Zietsch BP, Verweij KJH, Heath AC, Martin NG (2011) Variation in human mate choice: simultaneously investigating heritability, parental influence, sexual imprinting and assortative mating. Am Nat 177(5):605–616. doi:10.1086/659629
Zietsch BP, Verweij KJH, Burri AV (2012) Heritability of preferences for multiple cues of mate quality in humans. Evolution 66:1762–1772
Zietsch BP, Lee AJ, Sherlock JM, Jern P (2015) Variation in women’s facial masculinity preference is better explained by genetic differences than by previously identified context-dependent effects. Psychol Sci. doi:10.1177/0956797615591770
Acknowledgments
This study was funded by joint grants from the National Institutes of Health (Grant Numbers: AA07535, AA07728, AA10249, AA11998, MH31392) and the National Health and Medical Research Council (Australia, Grant Numbers: 941177 and 971232). James M. Sherlock is supported by an Australian Postgraduate Award. We also wish to thank Drew Bailey for integral input in developing a measure of the heritability of assortative mating.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
James M. Sherlock, Karin J. H. Verweij, Sean C. Murphy, Andrew C. Heath, Nicholas G. Martin, Brendan P. Zietsch declare no conflict of interests.
Ethical Approval
All research was conducted in accordance with the guidelines of the Queensland Institute of Medical Research Ethics Committee with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Edited by Carol Van Hulle.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sherlock, J.M., Verweij, K.J.H., Murphy, S.C. et al. The Role of Genes and Environment in Degree of Partner Self-Similarity. Behav Genet 47, 25–35 (2017). https://doi.org/10.1007/s10519-016-9808-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10519-016-9808-0