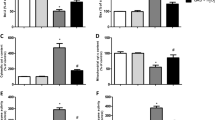
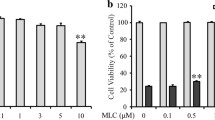
Abstract
Oxidative stress has been implicated in the etiology of neurodegenerative diseases and aging. Indeed, accumulation of reactive oxygen species, such as hydrogen peroxide, generated by inflammatory cells, leads to oxidative stress, which may contribute to the neuronal degeneration observed in a wide variety of neurodegenerative disorders of the central nervous system, such as Alzheimer’s disease. The present study indicates that H2O2-induced cell death can be inhibited in the presence of 1,2,4-triazine derivatives, as measured by MTT and caspase-3 activity. We further show that these compounds exert their protective effect by up-regulation of hemeoxygenase-1, glutamylcysteine synthetase, glutathione peroxidase and nuclear factor-erythroid 2 p45-related factor 2 (Nrf2), while they inhibit NF-κB and decrease lipid peroxidation. It shows that there is a potential cross talk between NF-κB and Nrf2, an important cytoprotective transcription factor in the presence of these compounds. Moreover, in order for drugs to be effective in the treatment of neurodegenerative diseases, they must be capable of penetrating the blood–brain barrier, whereas more than 98% of all potential central nervous system drugs don’t cross. Using a reliable model based on the artificial neural network indicated that these compounds satisfy this requirement.










Similar content being viewed by others
Abbreviations
- Aβ:
-
Amyloid β
- AD:
-
Alzheimer’s disease
- ANN:
-
Artificial neural network
- AREs:
-
Antioxidant responsive elements
- BBB:
-
Blood–brain barrier
- CAT:
-
Catalase
- CNS:
-
Central nervous system
- CSBP:
-
Plasma protein binding ratio
- ECL:
-
Electrochemiluminescence
- γ-GCS:
-
γ-Glutamylcysteine synthetase
- GPx-1:
-
Glutathione peroxidase
- GSH:
-
Glutathione
- HBA:
-
Abraham’s hydrogen-bond acidity
- HBB:
-
Hydrogen-bond basicity
- HO-1:
-
Hemeoxygenase-1
- H2O2 :
-
Hydrogen peroxide
- Keap1:
-
Kelch-like ECH-associated protein 1
- MDA:
-
Malondialdehyde
- MTT:
-
3-[4,5-dimethylthiazol-2-yl]-2,5-dephenyl tetrazolium bromide
- NF-κB:
-
Nuclear factor-κB
- NGF:
-
Nerve growth factor
- NRB:
-
Number of rotable bonds
- Nrf2:
-
Nuclear factor-erythroid 2 p45-related factor 2
- P-gp (H):
-
High affinity p-gp substrate probability
- PSA:
-
Polar surface area
- RMS:
-
Root-mean-sequare
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
References
Gotz ME, Kunig G, Riederer P, Youdim MB (1994) Oxidative stress: free radical production in neural degeneration. Pharmacol Ther 63:37–122
Butterfield DA, Drake J, Pocernich CB, Castegna A (2001) Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends Mol Med 7:548–554
Behl C, Davis JB, Lesley R, Schubert D (1994) Hydrogen peroxide mediates amyloid beta protein toxicity. Cell 77:817–827
Halliwell B, Aruoma OI (1991) DNA damage by oxygene-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett 281:9–19
Yoshikawa A, Saito Y, Maruyama K (2006) Lignan compounds and 4,4′-dihydroxybiphenyl protect C2C12 cells against damage from oxidative stress. Biochem Biophys Res Commun 344:394–399
Janssen-Heininger YM, Poynter ME, Baeuerle PA (2000) Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radic Biol Med 28(9):1317–1327
Akama KT, Van Eldik LJ (2000) Beta-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1beta- and tumor necrosis factor-alpha (TNFalpha)-dependent, and involves a TNFalpha receptor-associated factor- and NFkappaB-inducing kinase-dependent signaling mechanism. J Biol Chem 275(11):7918–7924
Bekircan O, Küxük M, Kahveci B, Kolayli S (2005) Convenient synthesis of fused heterocyclic 1,3,5-triazines from some N-acyl imidates and heterocyclic amines as anticancer and antioxidant agents. Arch Pharm (Weinheim) 338:365–372
Saxena S, Verma M, Saxena AK, Shanker K (1994) Triazines as anti-inflammatory agents. Arzneimittelforschung 44(6):766–769
Iwashita A, Maemoto T, Nakada H, Shima I, Matsuoka N, Hisajima H (2003) A novel potent radical scavenger, 8-(4-fluorophenyl)-2-((2E)-3-phenyl-2-propenoyl)-1,2,3,4-tetrahydropyrazolo[5,1-c] [1,2,4]triazine (FR210575), prevents neuronal cell death in cultured primary neurons and attenuates brain injury after focal ischemia in rats. J Pharmacol Exp Ther 307(3):961–968
Kim W, Kim Y, Min J, Kim DJ, Chang YT, Hecht MH (2006) A high-throughput screen for compounds that inhibit aggregation of the Alzheimer’s peptide. ACS Chem Biol 1(7):461–469
Adams R, Marvel CS (1941) Benzoin. In: Gilman H (ed) Organic synthesis collective, vol I, 2nd edn. Wiley, New York, pp 94–95
Clark HT, Dreger EE (1941) Benzil. Org Syn Coll 1:87–88
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Kutuk O, Basaga H (2003) Aspirin prevents apoptosis and NF-kappaB activation induced by H2O2 in HeLa cells. Free Radic Res 37:1267–1276
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Meth Enzymol 186:421–431
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Ind J Biochem Biophys 21:130–132
Aebi H (1984) Catalase in vitro. Meth Enzymol 105:121–126
Norinder U, Haeberlein M (2002) Computational approaches to the prediction of the blood-brain distribution. Adv Drug Deliv Rev 54(3):291–313
Garg P, Verma J (2006) In silico prediction of blood brain barrier permeability: an Artificial Neural Network model. J Chem Inf Model 46(1):289–297
Tamango E, Bardini P, Obbili A, Vitali A, Borghi R, Zaccheo D (2002) Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis 10:279–288
Miller DK (1997) The role of caspase family of cysteine proteases in apoptosis. Semin Immunol 9:35–49
Chen L, Liu L, Yin J, Luo Y, Huang S (2009) Hydrogen peroxide-induced neuronal apoptosis is associated with inhibition of protein phosphatase 2A and 5, leading to activation of MAPK pathway. Int J Biochem Cell Biol 41:1284–1295
Kim JW, Li MH, Jang JH, Na HK, Song NY, Lee C, Johnson JA, Surh YJ (2008) 15-Deoxy-Δ12, 14-prostaglandin J2 rescues PC12 cells from H2O2-induced apoptosis through Nrf2-mediated upregulation of heme oxygenase-1: potential roles of Akt and ERK1/2. Biochem Pharm 76:1577–1589
Kalayarasan S, Prabhu PN, Sriram N, Manikandan R, Arumugam M, Sudhandiran G (2009) Diallyl sulfide enhances antioxidants and inhibits inflammation through the activation of Nrf2 against gentamicin-induced nephrotoxicity in Wistar rats. Eur J Pharmacol 606:162–171
Tenhunen R, Marver HS, Schmid R (1969) Microsomal heme oxygenase characterization of the enzyme. J Biol Chem 244:6388–6394
Maines MD (1997) The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol 37:517–554
Ponka P (1999) Cell biology of heme. Am J Med Sci 318:241–256
Ogborne RM, Rushworth SA, Charalambos CA, O’Connell MA (2004) Heme oxygenase-1: a target for dietary antioxidants. Biochem Soc Trans 32:1003–1005
McNally SJ, Harrison EM, Ross JA, Garden OJ, Wigmore SJ (2007) Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int J Mol Med 19:165–172
Nguyen T, Sherratt PJ, Pickett CB (2003) Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol 43:233–260
Wild AC, Moinova HR, Mulcahy RT (1999) Regulation of γ-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J Biol Chem 274:33627–33636
Lee J, Johnson JA (2004) An important role of Nrf2-ARE pathway in cellular defense mechanism. J Biochem Mol Biol 37:139–143
Sun X, Erb H, Murphy TH (2005) Coordinate regulation of glutathione metabolism in astrocytes by Nrf2. Biochem Biophys Res Commun 326:371–377
Guroff G (1985) PC12 cells as a model of neuronal differentiation. In: Bottenstein JE (ed) Cell culture in neurosciences. Plenum Press, New York, pp 245–272
Feinstein DL, Galea E, Reis DJ (1997) Suppression of glial nitric oxide synthase induction by heat shock: effects on proteolytic degradation of IkappaB-alpha. Nitric Oxide 1:167–176
Guzhova IV, Darieva ZA, Melo AR, Margulis BA (1997) Major stress protein Hsp70 interacts with NF-κB regulatory complex in human T-lymphoma cells. Cell Stress Chaperones 2:132–139
Ran R, Lu A, Zhang L, Tang Y, Zhu H, Xu H, Feng Y, Han C, Zhou G, Rigby AC, Sharp FR (2004) Hsp70 promotes TNF-mediated apoptosis by binding IKK gamma and impairing NF-kappa B survival signaling. Genes Dev 18:1466–1481
Kalmar B, Greensmith L (2009) Induction of heat shock proteins for protection against oxidative stress. Adv Drug Deliv Rev 61:310–318
Jang JH, Surh YJ (2005) Beta-amyloid-induced apoptosis is associated with cyclooxygenase-2 up-regulation via the mitogen-activated protein kinase-NF-kappaB signaling pathway. Free Radic Biol Med 38(12):1604–1613
Kutuk O, Basaga H (2003) Aspirin prevents apoptosis and NF-kappaB activation induced by H2O2 in Hela cells. Free Radic Res 37(12):1267–1276
Ruiz-Ramos R, Lopez-Carrillo L, Rios-Perez AD, De Vizcaya-Ruíz A, Cebrian ME (2009) Sodium arsenite induces ROS generation, DNA oxidative damage, HO-1 and c-Myc proteins, NF-kappaB activation and cell proliferation in human breast cancer MCF-7 cells. Mutat Res 674(1–2):109–115
Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R (2003) Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J 371:887–895
Scapagnini G, Colombrita C, Amadio M, D’Agata V, Arcelli E, Sapienza M, Quattrone A, Calabrese V (2006) Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxid Redox Signal 8:395–403
Landis GN, Tower J (2005) Superoxide dismutase evolution and life span regulation. Mech Ageing Dev 126(3):365–379
Miller G (2002) Drug targeting. Breaking down barriers. Science 297(5584):1116–1118
Jeong WS, Kim IW, Hu R, Kong AN (2004) Modulatory properties of various natural chemopreventive agents on the activation of NF-kappaB signaling pathway. Pharm Res 21(4):661–670
Eftekharzadeh B, Khodagholi F, Abdi A, Maghsoudi N (2010) Alginate protects NT2 neurons against H2O2-induced neurotoxicity. Carbohydr Polym 79:1063–1072
Khodagholi F, Eftekharzadeh B, Maghsoudi N, Rezaei PF (2010) Chitosan prevents oxidative stress-induced amyloid beta formation and cytotoxicity in NT2 neurons: involvement of transcription factors Nrf2 and NF-kappaB. Mol Cell Biochem 337(1–2):39–51
Liu GH, Qu J, Shen X (2008) NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim Biophys Acta 1783(5):713–727
Acknowledgments
F. Khodagholi And M. Amini thank National Elite Fund, Iran, for the award of Young Scientist Research Fellowship. This work was supported partially by Shahid Beheshti University of Medical Sciences Research Funds and Tehran University of Medical Sciences Research Council. The authors thank Fatemeh Shaerzadeh for her excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tusi, S.K., Ansari, N., Amini, M. et al. Attenuation of NF-κB and activation of Nrf2 signaling by 1,2,4-triazine derivatives, protects neuron-like PC12 cells against apoptosis. Apoptosis 15, 738–751 (2010). https://doi.org/10.1007/s10495-010-0496-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-010-0496-6