Abstract
Little research has been done on egg diapause and the embryonic development of water mites. The aim of this study was to check the impact of temperature and periods of light on hatching of larvae of Eylais extendens. Three batches of eggs which were spawned on 30 July were placed at one of three temperatures (4, 10 and 20 °C) and two periods of light (7 and 14 h per day). Egg hatching (both, percentage of hatched larvae and rate of hatching) was found to differ between 4 versus 10 °C and between 4 versus 20 °C, but not between 10 versus 20 °C. The periods of light had no influence on hatching. This synchronization of hatching, enabling the eggs to emerge from diapause in the spring, could be considered an evolutionary adaptation aimed at postponing hatching of late-spawned eggs until a time allowing for completion of the full development cycle, including the parasitic larval stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Little research has been done on the embryonic development of water mites and the impact of environmental parameters on hatching time and numbers. Most research was involved with the number of eggs spawned and the hatching time specific to particular species of water mites and the environmental determinants of these processes (Davids 1973; Mayer 1985; Daszkiewicz and Zawal 2004; Włodarczyk and Zawal 2004; Martin 2010; Dzierzgowska et al. 2011; Kłosowska et al. 2011; Cichocka et al. 2015; Bańkowska et al. 2016), whereas only two studies concerned the influence of physicochemical parameters on hatching time and numbers (Rousch et al. 1997; Martin 2010). Egg diapause has often been reported for mites in general (Walter and Proctor 1999), but has been recorded much less frequently for Trombidia (Sabori and Zhang 1996; Sabori and Kamali 1999), and only a few observations pertain to water mites (Nielsen and Davids 1975; Gerecke 2002; Martin 2010; Smith et al. 2010).
The vast majority of water mite species parasitize aquatic insects. The hosts are usually Diptera species, but other hosts include Colembola, Plecoptera, Odonata, Heteroptera, Trichoptera and Coleoptera (Smith and Olivier 1976; Boehle 1996; Baker et al. 2007, 2008; Zawal and Szlauer-Łukaszewska 2012; Zawal and Buczyński 2013: Buczyńska et al. 2015; Stryjecki et al. 2015). Water mites of the genera Hydrovolzia, Piersigia, Hydryphantes, Limnochares, Eylais and Hydrachna parasitize Heteroptera and Coleoptera (Smith and Olivier 1976). Eylais and Hydrachna are the only two genera that parasitize both Heteroptera and Coleoptera; these water mites have the longest parasitic period, which allows them to survive in the parasitic larval stage in unfavourable environmental conditions, including the winter (Nielsen and Davids 1975; Zawal 2002, 2003a). Species of the genus Eylais may also hibernate in the form of eggs; this applies in particular to E. extendens and E. infundibulifera (Nielsen and Davids 1975).
Additionally, it has been observed that larvae spawning in late summer failed to hatch despite completing their embryonic development (fully formed larvae were visible under the egg integument). Water mites of the genus Eylais hibernate mainly in the form of parasitic larvae (Zawal 2003a, b, c; Davids et al. 2006). Eylais extendens parasitizes Coleoptera, and both small and large larvae have been recorded on hosts from April to November, which indicates that the hosts are infected over the entire growing period. This species is considered to be bivoltine and hibernates as parasitic larvae and eggs (Nielsen and Davids 1975; Zawal 2003a). Observations of interrupted development of E. extendens eggs laid in late summer confirm earlier suppositions regarding hibernation of eggs (Nielsen and Davids 1975), which would allow this standing-water species to survive the winter. This raises the question: what stimulus activates later development after the hibernation period, and what environmental factors affect the number of eggs hatched? We hypothesized that temperature and/or period of light affect the percentage of hatched larvae.
Materials and methods
Eggs were used, obtained from three female specimens of E. extendens caught in the first half of July. The female water mites were placed in 100-ml beakers. Eggs were laid after about 2 weeks. Egg-laying of each female lasted about 1–3 days, and each female laid 1–3 batches. About 3 weeks after the eggs were laid, fully developed larvae could be seen under the egg integuments, and some of them made small movements. In another case, where 14 batches of eggs were laid by five females in the spring or early summer, the larvae hatched when they were fully developed. Now, when eggs were laid in July, development of the larvae ceased: they stopped moving and did not hatch. The integuments remained transparent, the eggs did not become mouldy. For about 5 months the eggs were stored at about 20 °C until early January, the experiment began. The water used for breeding and experiment came from the collection site and was not exchanged during the experiment.
The batches contained several hundred eggs. Each batch was divided into six groups of about 50–90 eggs. Some of the eggs were damaged during the separation into groups. The damaged eggs turned white after a few days, which clearly distinguished them from the undamaged eggs. The eggs were placed at one of three temperatures (4, 10 or 20 °C), with two groups per temperature, and two periods of light (7 or 14 h per day), with one group per temperature. The experiment lasted 14 days, after which all eggs were placed at 20 °C with 14 h of illumination.
This experiment was conducted twice, in January and in April. In the experiment performed in January no larvae hatched. All eggs were kept under normal room conditions until April and then the experiment was repeated. This time the larvae hatched. The influence of temperature and period of light on hatching percentages was tested by Mann–Whitney U test (Statistica 12 PL).
Results
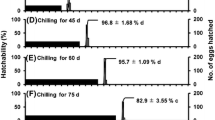
Temperature was found to affect the percentage of hatched larvae and the hatching time. About 60% of all eggs kept at 4 °C hatched, but only 7% of those exposed to other temperatures (although in one sample kept at 20 °C ca. 60% of the larvae hatched) (Fig. 1). Pairwise comparison indicated significant differences in % egg hatching at 4 versus 10 °C (Mann–Whitney U test: Z = 2.808, p = 0.005) and 4 versus 20 °C (Z = 2.088, p = 0.037), but not between 10 and 20 °C (Z = − 0.736, p = 0.46). The periods of light had no influence on the hatching percentage (4 °C: Z = 0.436, p = 0.66; 10 °C: Z = − 0.221, p = 0.82; 20 °C: Z = 0.221, p = 0.82) (Fig. 1).
The average hatching time was clearly shorter at 4 °C (about 7 days), than at 10 (ca. 14 days) or 20 °C (ca. 15 days) (Mann–Whitney U test, 4 vs. 10 °C: Z = 5.179, p < 0.000001; 4 vs. 20 °C: Z = 4.330, p = 0.000015; 10 vs. 20 °C: Z = − 0.751, p = 0.45) (Fig. 2). Again, the periods of light had no influence (4 °C: Z = − 0.384, p = 0.70; 10 °C: Z = − 0.627, p = 0.53; 20 °C: Z = − 0.626, p = 0.53).
Coming from 4 °C, a relatively large number of larvae (about 10) hatched just 1 day after the eggs had been placed at 20 °C. When hatching was complete (day 7) ca. 50 larvae had hatched. When eggs had been exposed to 10 or 20 °C, larvae began to appear after 6–8 days (Fig. 2).
Discussion
Our results confirm the existence of egg diapause in E. extendens, a standing-water species—egg diapause and delay of larval hatching has previously been reported for lotic species (Gerecke 2002; Martin 2010). The results clearly indicate temperature as the factor stimulating hatching of E. extendens larvae. A higher percentage (about 60%) of the eggs kept at 4 °C hatched, and they hatched faster: average hatching time was about 7 days. Of the eggs which were placed at 10 or 20 °C, fewer larvae hatched (about 7%) and they hatched slower: average hatching time was about 14 days. Only the winter temperature of 4 °C increased the hatching percentage, the intermediate temperature (10 °C) had the same effect as 20 °C.
According to Martin (2010), temperature stimulates eggs to emerge from diapause in water mites inhabiting springs and streams. Eggers (1995) showed a model of egg diapause based on Johnstoniana rapax, in which it is controlled by a chilling period of about 90 days at 5 °C followed by a rise in temperature.
The light–dark cycle, which simulated day length, had no influence on numbers of hatching larvae or hatching time. The results were the same for both 7 and 14 h photoperiod. An effect of lighting on the hatching rate of Sericostoma personatum (Trichoptera) was demonstrated by Wagner (1990), who found that longer exposure to light accelerated hatching.
In our first experiment, performed in January, no larvae hatched. This indicates that, although temperature enhances the metabolic rate and is a synchronising factor, it but does not function as a stimulus inducing hatching. Also day length does not appear to be such a factor. Apparently the main factor inducing hatching is the passage of time from spawning to hatching. But we do not know what constitutes the ‘interior clock’ of water mites. Perhaps the ‘interior clock’ is the change in duration of light exposure or chilling, followed by rising temperature. This synchronization of hatching, enabling eggs to emerge from diapause in the spring, could be considered an evolutionary adaptation aimed at postponing hatching of late-spawned eggs until a time allowing for completion of the full development cycle, including the parasitic stage of the larvae (Zawal 2003a, b).
According to Lanciani (1969) and Wohltmann (2001) Eylais spp. are multivoltine. Böttger (1962) demonstrated a bivoltine pattern in E. discreta which had two types of larvae: summer ones, which start engorgement as soon as attached to their host, and autumn ones, which start engorgement after hibernating on their host. The present investigation confirms E. extendens as a bivoltine species, as indicated by previous literature data (Nielsen and Davids 1975; Zawal 2002), with two ways of hibernating: as a parasitizing larva or as a diapausing egg with a fully developed larva waiting to hatch.
References
Baker RA, Mill PJ, Zawal A (2007) Mites on Zygoptera, with particular reference to Arrenurus species, selection sites and host preferences. Odonatologica 36:339–347
Baker RA, Mill PJ, Zawal A (2008) Ectoparasitic water mite larvae of the genus Arrenurus on the damselfly Coenagrion puella (Linnaeus) (Zygoptera: Coenagrionidae). Odonatologica 31:193–202
Bańkowska A, Kłosowska M, Gadawski P, Michoński G, Grabowski M, Pešić V, Zawal A (2016) Oviposition by selected water mite (Hydrachnidia) species from Lake Skadar and its catchment. Biologia 71:1027–1033. https://doi.org/10.1515/biolog-2016-0126
Boehle WR (1996) Contribution to the morphology and biology of larval Panisellus thinemanni (Viets, 1920) (Acari: Parasitengonae: Hydrachnidia). Acarologia 37:122–125
Böttger K (1962) Zur Biologie und Ethologie der einheimischen Wassermilben Arrenurus (Megaluracarus) globator (Muell.), 1776, Piona nodata nodata (Muell.), 1776 und Eylais infundibulifera meridionalis (Thon), 1899 (Hydrachnellae, Acari). Zool Jahrb Syst 89:501–584
Buczyńska E, Buczyński P, Zawal A, Michoński G, Szlauer-Łukaszewska A (2015) First record of parasitism of water mite larva (Acari: Hydrachnidia) on the pupa of Trichoptera. Acta Parasitol 60:196–199. https://doi.org/10.1080/01650424.2014.971816
Cichocka M, Biesiadka E, Pusz E (2015) Reproductive parameters of four species of water mites (Acari: Hydrachnidia). Biologia 70:1393–1400. https://doi.org/10.1515/biolog-2015-0154
Daszkiewicz M, Zawal A (2004) Płodność kilku gatunków wodopójek z podrodzajów Megaluracarus i Micruracarus w kontekście przystosowania do zmieniających się warunków środowiska. In: Ciaciura M (ed) Stan środowiska przyrodniczego podstawowym warunkiem zdrowotności społeczeństwa. Optimex, Fleurieux-sur-l’Arbresl, pp 361–368
Davids C (1973) The water mite Hydrachna conjecta Koenike, 1895 (Acari, Hydrachnellae), bionomics and relation to species of Corixidae (Hemiptera). Neth J Zool 23:363–429
Davids C, Di Sabatino A, Gerecke R, Gledhill T, Smit H, van der Hammen H (2006) Acari: hydrachnidia. In: Gerecke R (ed) Freshwater Fauna of Central Europe, vol 7/2-1. Spektrum Akademischer Verlag, München, pp 241–388
Dzierzgowska K, Zawal A, Stojanovski S (2011) Składanie jaj przez niektóre gatunki wodopójek (Hydrachnidia) z terenu Macedonii. In: Wysocki D, Kaliciuk J, Sadanowicz P (eds) Ogólnopolska Konferencja „Zwierzęta w życiu człowieka” oraz XX Jubileuszowy Zjazd Polskiego Towarzystwa Zoologicznego. P.P.H. ZAPOL Dmochowski, Sobczyk Sp. j., Szczecin, pp 42–48
Eggers A (1995) Observations on parasitism and development of Johnstoniana sp. (Prostigmata: Parasitengonae: Johnstonianidae). In: Kropczynska D, Boczek J, Tomczyk A (eds) The acari: physiological and ecological aspects of acari-host relationships. Oficina Dabor, Warsaw, pp 487–496
Gerecke R (2002) The water mites (Acari, Hydrachnidia) of a little disturbed forest stream in southwest Germany—a study on seasonality and habitat preference, with remarks on diversity patterns in different geographical areas. In: Bernini F, Nannelli G, Nuzzaci G, de Lillo E (eds) Acarid phylogeny and evolution. Adaptations in mites and ticks. Kluwer Academic, Dordrecht, pp 69–89
Kłosowska M, Bańkowska A, Zawal A (2011) Składanie jaj przez niektóre gatunki wodopójek (Hydrachnidia) z rzeki Krąpieli i jej zbiorników dolinnych. In: Wysocki D, Kaliciuk J, Sadanowicz P (eds) Ogólnopolska Konferencja „Zwierzęta w życiu człowieka” oraz XX Jubileuszowy Zjazd Polskiego Towarzystwa Zoologicznego. P.P.H. ZAPOL Dmochowski, Sobczyk Sp. j., Szczecin, pp 60–65
Lanciani CA (1969) Three species of Eylais (Acari: Eylaidae) parasitic on aquatic Hemiptera. Am Microsc Soc Trans 88:356–365
Martin P (2010) Observations on reproduction, development, and sexual behaviour of stream-inhabiting water mites (Acari: Hydrachnidia). In: Sabelis MW, Bruin J (eds) Trends in acarology, proceedings of the 12th international congress. Springer, New York, pp 303–312. https://doi.org/10.1007/978-90-481-9837-5
Mayer E (1985) Der Entwicklungszyklus von Hydrodroma despiciens (O.F. Müller 1776) (Acari: Hydrodromidae). Arch Hydrobiol Suppl 66:321–453
Nielsen GJ, Davids C (1975) Contribution to the knowledge of the morphology and biology of the larvae of four European Eylais species (Acari, Hydrachnellae). Acarologia 17:519–528
Rousch JM, Simmons TW, Kerans BL, Smith BP (1997) Relative acute of low pH and high iron on the hatching and survival of the water mite (Arrenurus manubriator) and the aquatic insect (Chronomus riparius). Environ Toxicol Chem 10:2144–2150
Sabori A, Kamali K (1999) Biology of Allothrombium shirazicum Zhang (Acari: Trombidiidae) in Garmsar, Semnan province, Iran. Syst Appl Acarol 4:199–200
Sabori A, Zhang Z-Q (1996) Biology of Allothrombium pulvinum Ewing (Acari: Trombidiidae) in West Mazandran, Iran. Exp Appl Acarol 20:137–142
Smith IM, Oliver DR (1976) The parasitic associations of larval water mites with imaginal aquatic insects, especially Chironomidae. Can Entomol 108:1427–1442
Smith IM, Cook DR, Smith BP (2010) Water mites (Hydrachnidiae) and other arachnids. In: Thorp JH, Covich AP (eds) Ecology and classification of North American freshwater invertebrates, 3rd edn. Elsevier, Amsterdam, pp 485–585
Stryjecki R, Zawal A, Gadawski P, Buczyńska E, Buczyński P (2015) New host-parasite associations of Hydrachnidia (Acari) on Chironomidae (Diptera) from Poland. Biologia Sect Zool 70:1210–1214. https://doi.org/10.1515/biolog-2015-0136
Wagner R (1990) A laboratory study on the life cycle of Sericostoma personatum (Kirby & Spence), and light dark-dependent food consumption. Hydrobiologia 208:201–212
Walter DE, Proctor HC (1999) Mites—ecology, evolution and behaviour. CABI Publishing, Wallingford
Włodarczyk A, Zawal A (2004) Porównanie płodności kilku gatunków wodopójek z podrodzaju Arrenurus s. str. ze środowisk o różnej antropopresji. In: Ciaciura M (ed) Stan środowiska przyrodniczego podstawowym warunkiem zdrowotności społeczeństwa. Optimex, Fleurieux-sur-l’Arbresl, pp 361–368
Wohltmann A (2001) The evolution of life histories in Parasitengona (Acari; Prostigmata). Acarologia 41:145–204
Zawal A (2002) Parasitism of water mite (Hydrachnellae) larvae of genus Hydrachna on water beetles in Poland. Acarologia 42:361–370
Zawal A (2003a) Parasitism of water mite (Hydrachnellae) larvae of genus Eylais on water beetles in Poland. Acarologia 53:39–47
Zawal A (2003b) The role of insects in the dispersion of water mites. Acta Biol Univ Daugavp 3:9–14
Zawal A (2003c) Strategia rozwoju a sukces reprodukcyjny niektórych wodopójek (Hydrachnellae). In: Rogalska S, Domagała J (eds) Człowiek i środowisko przyrodnicze Pomorza Zachodniego. I Środowisko biotyczne. Uniwersytet Szczeciński, Szczecin, pp 135–137
Zawal A, Buczyński P (2013) Parasitism of Odonata by Arrenurus (Acari: Hydrachnidia) larvae in the Lake Świdwie, nature reserve (NW Poland). Acta Parasitol 58:486–495. https://doi.org/10.2478/s11686-013-0162-6
Zawal A, Szlauer-Łukaszewska A (2012) Water mite parasites (Hydrachnidia) of odonates from the nature reserve “Jezioro Szare”, northwestern Poland. Odonatologica 41:267–275
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zawal, A., Bańkowska, A. & Nowak, A. Influence of temperature and light–dark cycle on hatching of Eylais extendens. Exp Appl Acarol 74, 283–289 (2018). https://doi.org/10.1007/s10493-018-0238-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-018-0238-y