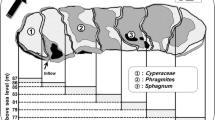
Abstract
Northern Chile harbors different bioclimatic zones including hyper-arid and arid ecosystems and hotspots of microbial life, such as high altitude wetlands, which may contribute differentially to greenhouse gases (GHG) such as carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O). In this study, we explored ground level GHG distribution and the potential role of a wetland situated at 3800 m.a.s.l, and characterized by high solar radiation < 1600 W m−2, extreme temperature ranges (−12 to 24 °C) and wind stress (< 17 m s−1). The water source of the wetland is mainly groundwater springs, which generates streams and ponds surrounded by peatlands. These sites support a rich microbial aquatic life including diverse bacteria and archaea communities, which transiently form more complex structures, such as microbial mats. In this study, GHG were measured in the water and above ground level air at the wetland site and along an elevation gradient in different bioclimatic areas from arid to hyper-arid zones. The microbiome from the water and sediments was described by high-throughput sequencing 16S rRNA and rDNA genes. The results indicate that GHG at ground level were variable along the elevation gradient potentially associated with different bioclimatic zones, reaching high values at the high Andean steppe and variable but lower values in the Atacama Desert and at the wetland. The water areas of the wetland presented high concentrations of CH4 and CO2, particularly at the spring areas and in air bubbles below microbial mats. The microbial community was rich (> 40 phyla), including archaea and bacteria potentially active in the different matrices studied (water, sediments and mats). Functional microbial groups associated with GHG recycling were detected at low frequency, i.e., < 2.5% of total sequences. Our results indicate that hyper-arid and arid areas of northern Chile are sites of GHG exchange associated with various bioclimatic zones and particularly in aquatic areas of the wetland where this ecosystem could represent a net sink of N2O and a source for CH4 and CO2.





Similar content being viewed by others
References
Aceituno P (1997) Aspectos generales del clima en el altiplano sudamericano. In: Charrier R, Aceituno P, Castro M, Llanos A, Raggi LA (eds) El Altiplano: ciencia y conciencia de los Andes. Actas del segundo Simposio internacional de Estudios Altiplánicos, Santiago, pp 63–69
Aguilar P, Acosta E, Dorador C, Sommaruga R (2016) Large differences in bacterial community composition among three nearby extreme waterbodies of the high Andean plateau. Front Microbiol. https://doi.org/10.3389/fmicb.2016.00976
Albarracín VH, Kurth D, Ordoñez OF et al (2015) High-up: a remote reservoir of microbial extremophiles in central Andean Wetlands. Front Microbiol 6:1404. https://doi.org/10.3389/fmicb.2015.01404
Atlas EL, Gordon LI, Hager SW, Park PK (1971) A practical manual for use of the Technicon AutoAnalyzer in seawater nutrient analyses (revised). Tech. Rep. 215, Department of Oceanography, School of Science, Oregon State University, Corvallis
Barrett BS, Campos DA, Veloso JV, Rondanelli R (2016) Extreme temperature and precipitation events in March 2015 in central and northern Chile. J Geophys Res Atmos 121:4563–4580. https://doi.org/10.1002/2016JD024835
Barros N, Feijóo S, Salgado J et al (2008) The dry limit of microbial life in the Atacama desert revealed by calorimetric approaches. Eng Life Sci 8:477–486. https://doi.org/10.1002/elsc.200820236
Bridgham SD, Cadillo-Quiroz H, Keller JK, Zhuang Q (2013) Methane emissions from wetlands: biogeochemical, microbial, and modeling perspectives from local to global scales. Glob Chang Biol 19:1325–1346. https://doi.org/10.1111/gcb.12131
Cornejo M, Farias L, Paulmier A (2006) Temporal variability in N2O water content and its air-sea exchange in an upwelling area off central Chile (36ºS). Mar Chem 101:85–94
DeBruyn JM, Nixon LT, Fawaz MN et al (2011) Global biogeography and quantitative seasonal dynamics of Gemmatimonadetes in soil. Appl Environ Microbiol 77:6295–6300. https://doi.org/10.1128/AEM.05005-11
Ding WX, Cai ZC (2007) Methane emission from natural wetlands in China: summary of years 1995–2004. Pedosphere 17:475–486. https://doi.org/10.1016/S1002-0160(07)60057-5
Dorador C, Busekow A, Vila I et al (2008) Molecular analysis of enrichment cultures of ammonia oxidizers from the Salar de Huasco, a high altitude saline wetland in northern Chile. Extremophiles 12:405–414. https://doi.org/10.1007/s00792-008-0146-x
Dorador C, Vila I, Remonsellez F et al (2010) Unique clusters of Archaea in Salar de Huasco, an athalassohaline evaporitic basin of the Chilean Altiplano. FEMS Microbiol Ecol 73:291–302. https://doi.org/10.1111/j.1574-6941.2010.00891.x
Edgar RC, Haas BJ, Clemente JC et al (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. https://doi.org/10.1093/bioinformatics/btr381
Farias ME, Rasuk MC, Gallagher KL et al (2017) Prokaryotic diversity and biogeochemical characteristics of benthic microbial ecosystems at La Brava, a hypersaline lake at Salar de Atacama, Chile. PLoS ONE 12:1–25. https://doi.org/10.1371/journal.pone.0186867
Farías ME, Rascovan N, Toneatti DM et al (2013) The discovery of stromatolites developing at 3570 m above sea level in a high-altitude volcanic Lake Socompa, Argentinean Andes. PLoS One. https://doi.org/10.1371/journal.pone.0053497
Fernandez AB, Rasuk MC, Visscher PT et al (2016) Microbial diversity in sediment ecosystems (evaporites domes, microbial mats, and crusts) of Hypersaline Laguna Tebenquiche, Salar de Atacama, Chile. Front Microbiol 7:1–18. https://doi.org/10.3389/fmicb.2016.01284
Goreau TJ, Kaplan WA, Wofsy SC (1980) Production of NO2 − and N2O by nitrifying bacteria at reduced concentrations of oxygen. Appl Environ Microbiol 40:526–532
Hall SJ, Silver WL, Amundson R (2012) Greenhouse gas fluxes from Atacama Desert soils: a test of biogeochemical potential at the Earth’s arid extreme. Biogeochemistry 111:303–315. https://doi.org/10.1007/s10533-011-9650-7
He GX, Li KH, Liu XJ et al (2014) Fluxes of methane, carbon dioxide and nitrous oxide in an alpine wetland and an alpine grassland of the Tianshan Mountains, China. J Arid Land 6:717–724. https://doi.org/10.1007/s40333-014-0070-0
Hernández KL, Yannicelli B, Olsen LM et al (2016) Microbial activity response to solar radiation across contrasting environmental conditions in Salar de Huasco, northern Chilean altiplano. Front Microbiol 7:1–13. https://doi.org/10.3389/fmicb.2016.01857
Holmes RM, Aminot A, Kérouel R et al (1999) A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can J Fish Aquat Sci 56:1801–1808. https://doi.org/10.1139/f99-128
Houston J (2006) Variability of precipitation in the Atacama Desert: its causes and hydrological impact. Int J Climatol 26:2181–2198. https://doi.org/10.1002/joc.1359
Intergovernmental Panel on Climate Change (IPCC) (2007) Climate Change 2007: The physical science basis: working group I contribution to the fourth assessment report of the IPCC. Cambridge University Press. http://www.amazon.ca/exec/obidos/redirect?tag=citeulike09-20&
Kelley CA, Nicholson BE, Beaudoin CS et al (2014) Trimethylamine and organic matter additions reverse substrate limitation effects on the δ13c values of methane produced in hypersaline microbial mats. Appl Environ Microbiol 80:7316–7323. https://doi.org/10.1128/AEM.02641-14
Kulaev I, Kulakovskaya T (2000) Polyphosphate and phosphate pump. Annu Rev Microbiol 54:709–734. Review https://doi.org/10.1146/annurev.micro.54.1.709
Li T, Zhang Q, Cheng Z et al (2016) Modeling CH4 emissions from natural wetlands on the Tibetan Plateau over the past 60 years: influence of climate change and wetland loss. Atmosphere (Basel) 7:90. https://doi.org/10.3390/atmos7070090
Löscher CR, Kock A, Könneke M et al (2012) Production of oceanic nitrous oxide by ammonia-oxidizing archaea. Biogeosciences 9:2419–2429. https://doi.org/10.5194/bg-9-2419-2012
Luebert F, Pliscoff P (2006) Sinopsis bioclimática y vegetacional de Chile, 1st edn. Editorial Universitaria, p 323
Majumdar D, Rao P, Maske N (2017) Inter-seasonal and spatial distribution of ground-level greenhouse gases (CO2, CH4, N2O) over Nagpur in India and their management roadmap. Environ Monit Assess 189:121. https://doi.org/10.1007/s10661-017-5829-2
McAuliffe C (1971) Gas chromatographic determination of solutes by multiple phase equilibrium. Chem Technol 1:46–51
Merbt SN, Stahl DA, Casamayor EO et al (2012) Differential photoinhibition of bacterial and archaeal ammonia oxidation. FEMS Microbiol Lett 327:41–46. https://doi.org/10.1111/j.1574-6968.2011.02457.x
Molina V, Hernández K, Dorador C et al (2016) Bacterial active community cycling in response to solar radiation and their influence on nutrient changes in a high-altitude wetland. Front Microbiol 7:1–15. https://doi.org/10.3389/fmicb.2016.01823
Navarro-González R, Rainey FA, Molina P, et al (2003) Mars-like soils in the Atacama Desert, Chile, and the dry limit of microbial life. Science (80-) 302:1018–1021. https://doi.org/10.1126/science.1089143
Olson RJ (1980) Nitrate and ammonium uptake in Antarctic waters1. Limnol Oceanogr 25:1064–1074. https://doi.org/10.4319/lo.1980.25.6.1064
Quade J, Rech JA, Latorre C et al (2007) Soils at the hyperarid margin: the isotopic composition of soil carbonate from the Atacama Desert, Northern Chile. Geochim Cosmochim Acta 71:3772–3795. https://doi.org/10.1016/j.gca.2007.02.016
Quast C, Pruesse E, Yilmaz P et al (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. https://doi.org/10.1093/nar/gks1219
Schloss PD, Westcott SL, Ryabin T et al (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. https://doi.org/10.1128/AEM.01541-09
Schreiber F, Wunderlin P, Udert KM, Wells GF (2012) Nitric oxide and nitrous oxide turnover in natural and engineered microbial communities: biological pathways, chemical reactions, and novel technologies. Front Microbiol. https://doi.org/10.3389/fmicb.2012.00372
Stevens H, Ulloa O (2008) Bacterial diversity in the oxygen minimum zone of the eastern tropical South Pacific. Environ Microbiol 10:1244–1259. https://doi.org/10.1111/j.1462-2920.2007.01539.x
Torres R, Turner D, Rutllant J, Sobarzo M, Antezana T, González HE (2002) CO2 outgassing off Central Chile (31–30°S) and northern Chile (24–23°S) during austral summer 1997: The effect of wind intensity on the upwelling and ventilation of CO2-rich waters. Deep Sea Res. Part I 49:1413–1429. https://doi.org/10.1016/s09670637(02)00034-1
Wan W, Li H, Xie H et al (2017) A comprehensive data set of lake surface water temperature over the Tibetan Plateau derived from MODIS LST products 2001–2015. Sci Data 4:170095
Wuebbles DJ, Hayhoe K (2002) Atmospheric methane and global change. 57:177–210
Zhang H, Sekiguchi Y, Hanada S et al (2003) Gemmatimonas aurantiaca gen. nov., sp. nov., a Gram-negative, aerobic, polyphosphate-accumulating micro-organism, the first cultured representative of the new bacterial phylum Gemmatimonadetes phyl. nov. Int J Syst Evol Microbiol 53:1155–1163. https://doi.org/10.1099/ijs.0.02520-0
Acknowledgements
We are grateful to M.J. Gálvez and D. Kalenitchenko for their technical support, to all the participants of the field trips and particularly to Pedro and Margarita Luca for their hospitality at Salar de Huasco shelter facilities. This work is part of the FONDECYT Project #1140356, #1140179, #11130418, #1171324, #1150891, LIA-MORFUN, COPAS Sur-Austral (PFB-31), INCAR center (1510027), and CONICYT-CNRS 2014 France (PCCI140034).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Molina, V., Eissler, Y., Cornejo, M. et al. Distribution of greenhouse gases in hyper-arid and arid areas of northern Chile and the contribution of the high altitude wetland microbiome (Salar de Huasco, Chile). Antonie van Leeuwenhoek 111, 1421–1432 (2018). https://doi.org/10.1007/s10482-018-1078-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-018-1078-9