Abstract
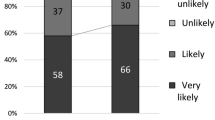
We analyzed data from 1428 users of the dapivirine vaginal ring, who participated in the MTN-020/ASPIRE phase III trial and subsequent open-label extension MTN-025/HOPE trial, to examine relationships between perceived ring protection, social disclosures, and self-reported ring adherence. In HOPE, 77% perceived the ring to be highly effective, and this view was associated with speaking: (a) to a greater number of people about the study, (b) with other participants, (c) to more people who were in favor of the ring, and (d) to more people whose opinions were valued. Reported adherence was not directly associated with perceived protection but was associated with disclosing to someone who was in favor of the ring. These findings suggest the importance of women’s internalized ideas about the protective benefits of the DVR in sharing information about the ring and the importance of social support on adherence.

Similar content being viewed by others
Data Availability
Data are available through the Microbicide Prevention Trials Network.
Code Availability
Code for this project is available by contacting the corresponding author.
References
Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410.
Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. 2016;375:2121–32.
Nel A, Van Niekerk N, Kapiga S, Bekker LG, Gama C, Gill K, et al. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med. 2016;375(22):2133–43.
Browne E, Hendrix CW, van der Straten A, Kiweewa FM, Mgodi NM, Palanee-Phillips T, et al. Greater dapivirine release from the dapivirine vaginal ring is correlated with lower risk of HIV-1 acquisition. JIAS. 2020;23:e25634.
Baeten JM, Haberer JE, Liu AY, Sista N. Preexposure prophylaxis for HIV prevention: where have we been and where are we going? J Acquir Immune Defic Syndr. 2013. https://doi.org/10.1097/QAI.0b013e3182986f69.
Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African Women. N Engl J Med. 2015;372:509–18. https://doi.org/10.1056/NEJMoa1402269 ([Internet]).
Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–22. Available from http://www.ncbi.nlm.nih.gov/pubmed/22784040
Katz AWK, Naidoo K, Reddy K, Chitukuta M, Nabukeera J, Siva S, et al. The power of the shared experience: MTN-020/ASPIRE trial participants’ descriptions of peer influence on acceptability of and adherence to the dapivirine vaginal ring for HIV prevention. AIDS Behav. 2020. https://doi.org/10.1007/s10461-020-02799-0.
Browne EN, Montgomery ET, Mansfield C, Boeri M, Mange B, Beksinska M, et al. Efficacy is not everything: eliciting women’s preferences for a vaginal HIV prevention product using a discrete-choice experiment. AIDS Behav. 2020;24(5):1443–51. https://doi.org/10.1007/s10461-019-02715-1.
Minnis AM, Browne EN, Boeri M, Agot K, Van Der Straten A, Ahmed K, et al. Young women’s stated preferences for biomedical HIV prevention: results of a discrete choice experiment in Kenya and South Africa. J Acquir Immune Defic Syndr. 2019;80(4):394–403. https://doi.org/10.1097/QAI.0000000000001945.
Golub SA, Gamarel KE, Rendina HJ, Surace A, Lelutiu-Weinberger CL. From efficacy to effectiveness: facilitators and barriers to PrEP acceptability and motivations for adherence among MSM and transgender women in New York City. AIDS Patient Care STDS. 2013;27(4):248–54. https://doi.org/10.1089/apc.2012.0419.
Young I, Flowers P, McDaid LM. Barriers to uptake and use of pre-exposure prophylaxis (PrEP) among communities most affected by HIV in the UK: findings from a qualitative study in Scotland. BMJ Open. 2014;4(11):e005717. https://doi.org/10.1136/bmjopen-2014-005717.
Koechlin FM, Fonner VA, Dalglish SL, O’Reilly KR, Baggaley R, Grant RM, et al. Values and preferences on the use of oral pre-exposure prophylaxis (PrEP) for HIV prevention among multiple populations: a systematic review of the literature. AIDS Behav. 2017;21(5):1325–35. https://doi.org/10.1007/s10461-016-1627-z.
Palanee-Phillips T, Roberts ST, Reddy K, Govender V, Naidoo L, Siva S, et al. Impact of partner-related social harms on women’s adherence to the dapivirine vaginal ring during a phase III trial. J Acquir Immune Defic Syndr. 2018;79(5):580–9. https://doi.org/10.1097/QAI.0000000000001866.
Roberts ST, Nair G, Baeten JM, Palanee-Philips T, Schwartz K, Reddy K, et al. Impact of male partner involvement on women’s adherence to the dapivirine vaginal ring during a phase III HIV prevention trial. AIDS Behav. 2020;24(5):1432–42. https://doi.org/10.1007/s10461-019-02707-1.
Montgomery ET, Van Der Straten A, Chitukuta M, Reddy K, Woeber K, Atujuna M, et al. Acceptability and use of a dapivirine vaginal ring in a phase III trial. AIDS. 2017;31(8):1159–67. https://doi.org/10.1097/QAD.0000000000001452.
van der Straten A, Ryan JH, Reddy K, Etima J, Taulo F, Mutero P, et al. Influences on willingness to use vaginal or oral HIV PrEP during pregnancy and breastfeeding in Africa: the multisite MAMMA study. J Int AIDS Soc. 2020;23(6):e25536. https://doi.org/10.1002/jia2.25536.
Wagner LD, Roberts ST, O’Rourke S, Celum C, Baeten JM, Bukusi E, et al. Challenges with oral pre-exposure prophylaxis (PrEP) disclosure among adolescent girls and young women (AGYW) in Kenya and South Africa. JAIDS Res Hum Retroviruses. 2018;34:373.
Golub SA, Fikslin RA, Goldberg MH, Peña SM, Radix A. Predictors of PrEP uptake among patients with equivalent access. AIDS Behav. 2019;23(7):1917–24. https://doi.org/10.1007/s10461-018-2376-y.
Peng P, Su S, Fairley CK, Chu M, Jiang S, Zhuang X, et al. A global estimate of the acceptability of pre-exposure prophylaxis for HIV among men who have sex with men: a systematic review and meta-analysis. AIDS Behav. 2018;22(4):1063–74. https://doi.org/10.1007/s10461-017-1675-z.
Calabrese SK, Dovidio JF, Tekeste M, Taggart T, Galvao RW, Safon CB, et al. HIV pre-exposure prophylaxis stigma as a multidimensional barrier to uptake among women who attend planned parenthood. J Acquir Immune Defic Syndr. 2018;79(1):46–53. https://doi.org/10.1097/QAI.0000000000001762.
Tangmunkongvorakul A, Chariyalertsak S, Rivet Amico K, Saokhieo P, Wannalak V, Sangangamsakun T, et al. Facilitators and barriers to medication adherence in an HIV prevention study among men who have sex with men in the iPrEx study in Chiang Mai, Thailand. AIDS Care. 2013;25(8):961–7. https://doi.org/10.1080/09540121.2012.748871.
Liu A, Cohen S, Follansbee S, Cohan D, Weber S, Sachdev D, et al. Early experiences implementing pre-exposure prophylaxis (PrEP) for HIV prevention in San Francisco. PLoS Med. 2014;11(3):e1001613. https://doi.org/10.1371/journal.pmed.1001613.
Baeten J, Palanee-Phillips T, Mgodi N, Mayo A, Nel A, Rosenberg Z, et al. High uptake and reduced HIV-1 incidence in an open-label trial of the dapivirine ring. In: Confrence on Retroviruses and Opportunistic Infections (CROI); 2018 Mar 4–7; Boston, Massachusetts.
Mensch BS, Richardson BA, Husnik M, Brown ER, Kiweewa FM, Mayo AJ, et al. Vaginal ring use in a phase 3 microbicide trial: a comparison of objective measures and self-reports of non-adherence in ASPIRE. AIDS Behav. 2019;23(2):504–12. https://doi.org/10.1007/s10461-018-2261-8.
Wilson IB, Lee Y, Michaud J, Fowler FJ, Rogers WH. Validation of a new three-item self-report measure for medication adherence. AIDS Behav. 2016;20(11):2700–8. https://doi.org/10.1007/s10461-016-1406-x.
Mensch BS, Brown ER, Liu K, Marrazzo J, Chirenje ZM, Gomez K, et al. Reporting of adherence in the VOICE trial: did disclosure of product nonuse increase at the termination visit? AIDS Behav. 2016;20(11):2654–61. https://doi.org/10.1007/s10461-016-1312-2.
International Partnership forMicrobicides. IPM’s dapivirine ring for women’s HIV prevention receives WHO prequalification. 2020 [cited 2020 Dec 8]. Available from https://www.ipmglobal.org/content/ipm’s-dapivirine-ring-women’s-hiv-prevention-receives-who-prequalification
Cairns GP, Race K, Goicochea P. PrEP: controversy, agency and ownership. J Int AIDS Soc. 2016. https://doi.org/10.7448/IAS.19.7.21120.
Koechlin FM, Fonner VA, Dalglish SL, O’Reilly KR, Baggaley R, Grant RM, et al. Values and preferences on the use of oral pre-exposure prophylaxis (PrEP) for HIV prevention among multiple populations: a systematic review of the literature. AIDS Behav. 2017;21:1325–35. Available from https://www.scopus.com/inward/record.uri?eid=2-s2.0-85000785127&doi=10.1007%2Fs10461-016-1627-z&partnerID=40&md5=cc47a59dd09f09e723e4cc32da4467ca
Gati Mirembe B, Cabrera MV, Cobbing M, Mgodi N, Palanee TP, Mayo A, Dadabhai S, Mansoor LE, Siva S, Nair G, Akello CA, Nakabiito C, Soto-Torres LE, van der Straten A, Baeten JM, et al. Correlates of dapivirine vaginal ring uptake and acceptance among women participating in an open label extension trial-MTN-025/HOPE. In: 4th HIV Res Prev Conf (HIVR4P). Virtual; 2020. https://programme.hivr4p.org/Abstract/Abstract/605.
Van Der Straten A, Brown ER, Marrazzo JM, Chirenje MZ, Liu K, Gomez K, et al. Divergent adherence estimates with pharmacokinetic and behavioural measures in the MTN-003 (VOICE) study. J Int AIDS Soc. 2016;19:20642.
Velloza J. The influence of HIV-related stigma on PrEP disclosure and adherence over time among AGYW in HPTN 082. Miami, Florida: IAPAC Adherence Conf; 2019.
Acknowledgements
ASPIRE/MTN-020 and HOPE/MTN-025 Study Team Leadership: Jared Baeten, University of Washington (Protocol Chair); Thesla Palanee-Phillips, Wits Reproductive Health and HIV Institute (Protocol Co-chair); Nyaradzo Mgodi, University of Zimbabwe College of Health Sciences Clinical Trials Unit (Protocol Co-chair); Elizabeth Brown, Fred Hutchinson Cancer Research Center (Protocol Statistician); Lydia Soto-Torres, US National Institute of Allergy and Infectious Diseases (Medical Officer); Katie Schwartz, FHI 360 (Clinical Research Manager), Ashley Mayo, FHI 360 (Clinical Research Manager).
ASPIRE/MTN-020 and HOPE/MTN-025 Study sites and site Investigators of Record: Malawi, Blantyre site (Johns Hopkins University, Queen Elizabeth Hospital): Bonus Makanani; Malawi, Lilongwe site (University of North Carolina, Chapel Hill): Francis Martinson, Lameck Chinula; South Africa, Cape Town site (University of Cape Town): Linda-Gail Bekker, Gonasagrie Nair; South Africa, Durban – Botha’s Hill, Chatsworth, Isipingo, Tongaat, Umkomaas, Verulam sites (South African Medical Research Council): Vaneshree Govender, Samantha Siva, Zakir Gaffoor, Logashvari Naidoo, Arendevi Pather, Nitesha Jeenarain, Gita Ramjee, Dishiki Kalonji, and Nishanta Singh; South Africa, Durban, eThekwini site (Center for the AIDS Programme for Research in South Africa): Gonasagrie Nair, Leila Mansoor; South Africa, Johannesburg site (Wits RHI): Thesla Palanee-Phillips; Uganda, Kampala site (John Hopkins University, Makerere University): Flavia Matovu, Brenda Gati; Zimbabwe, Chitungwiza and Harare—Zengeza, Seke South and Splihaus sites (University of Zimbabwe Clinical Trials Research Centre): Nyaradzo Mgodi, Portia Hunidzarira, and Felix Mhlanga.
Data management was provided by The Statistical Center for HIV/AIDS Research & Prevention (Fred Hutchinson Cancer Research Center, Seattle, WA) and site laboratory oversight was provided by the Microbicide Trials Network Laboratory Center (Pittsburgh, PA). The vaginal rings used in this study were developed and supplied by the International Partnership for Microbicides (IPM).
Jared Baeten, Thesla Palanee-Phillips, Nyaradzo Mgodi, Elizabeth Brown, Lydia Soto-Torres, Katie Schwartz, Ashley Mayo, Bonus Makanani, Francis Martinson, Lameck Chinula, Linda-Gail Bekker, Gonasagrie Nair, Vaneshree Govender, Samantha Siva, Zakir Gaffoor, Logashvari Naidoo, Arendevi Pather, Nitesha Jeenarain, Gita Ramjee, Dishiki Kalonji, Nishanta Singh, Leila Mansoor, Flavia Matovu, Brenda Gati, Portia Hunidzarira, and Felix Mhlanga.
Funding
The Microbicide Trials Network is funded by the National Institute of Allergy and Infectious Diseases (UM1AI068633, UM1AI068615, UM1AI106707), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the study participants as well as the ASPIRE/HOPE Study team members who implemented the trials.
Author information
Authors and Affiliations
Consortia
Contributions
MCDS, AvdS contributed to the conception, design of the analysis and writing of the paper. E.B. provided statistical support on the analysis. The remaining authors were involved in data acquisition, data collection, study management and design of the original parent study. All authors have reviewed the paper, provided comments and edits to the manuscript and have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We declare no competing interests.
Consent to Participate
All participants provided written informed consent.
Ethical Approval
The trial protocol was approved by the ethics review committee at each site.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Stoner, M.C.D., Brown, E.R., Palanee-Phillips, T. et al. The Influence of Perceived Dapivirine Vaginal Ring Effectiveness on Social Disclosure and Ring Adherence. AIDS Behav 25, 4169–4179 (2021). https://doi.org/10.1007/s10461-021-03286-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-021-03286-w