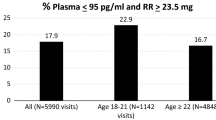
Abstract
Discrepancies between self-reported and actual adherence to biomedical HIV interventions is common and in clinical trials can compromise the integrity of findings. One solution is to monitor adherence biomarkers, but it is not well understood how to navigate biomarker feedback with participants. We surveyed 42 counselors and interviewed a subset of 22 to characterize their perspectives about communicating with participants about residual drug levels, an objective marker of adherence, within MTN-025/HOPE, a Phase 3b clinical trial of a vaginal ring to prevent HIV. When biomarkers indicated low drug levels that mismatched high adherence by self-report, counselors encountered barriers to acceptance and comprehension among participants. However, discrepancies between low self-report and higher drug levels generally stimulated candor. Women recollected times they had not used the product and disclosed problems that counselors thought might otherwise have remained forgotten or concealed. Navigating conversations toward HIV prevention was easier at mid-range drug levels and when women indicated motivation to prevent HIV. Ratings of residual drug level offered a somewhat objective measure of adherence and protection that counselors perceived as meaningful to participants and as a valuable catalyst for broaching conversation about HIV prevention. However, communication about drug levels required that counselors navigate emotional barriers, respond skillfully to questions about accuracy, and pivot conversations non-judgmentally away from numerical results and toward the priority of HIV prevention. Findings suggest a role for biomarker feedback in future clinical trials as well as other clinical contexts where biomarkers may be monitored, to motivate disclosure of actual adherence and movement toward HIV prevention.
Clinical Trial Number NCT02858037.
Resumen
Discrepancias entre la adherencia auto-reportada y la verdadera a intervenciones biomédicas de VIH pueden comprometer los ensayos clínicos. Una solución es monitorear la adherencia por medio de ensayos biológicos, pero no se entiende bien cómo comunicar estas medidas a los participantes. En MTN-025/HOPE, un ensayo fase 3b de un anillo vaginal para prevenir VIH, encuestamos a 42 consejeros de adherencia y entrevistamos a un subconjunto de 22 para caracterizar sus perspectivas sobre comunicar una medida objetiva de adherencia al anillo, el nivel residual de droga (RDL por sus siglas en inglés). Los consejeros reportaron que los participantes apreciaron la retroalimentación del RDL como una indicación de su protección de VIH. Niveles más altos de droga estimularon euforia y alivio mientras niveles mas bajos resultaron en desilusión. Una postura no crítica y el apoyo a la autonomía de elegir otras alternativas al anillo promovieron divulgación de las razones por la falta de adherencia. Hablar del monitoreo de RDL como “protección” en vez de “adherencia” ayudó a cambiar el enfoque desde resultados numéricos hasta la meta mayor del ensayo de prevenir el VIH. Personalizar la retroalimentación de medidas objetivas de adherencia requiere una conversación cuidadosa para minimizar las actitudes defensivas. La retroalimentación personalizada también se puede implementar de forma que motive la divulgación de la falta de adherencia y evoque un compromiso a prácticas de prevención. Enfatizar las motivaciones de las mujeres a prevenir el VIH, en vez de los resultados numéricos, puede incentivar a los usuarios consistentes a continuar y a los usuarios inconsistentes a usar métodos alternativos de prevención.

Similar content being viewed by others
References
Corneli AL, Deese J, Wang M, Taylor D, Ahmed K, Agot K, et al. FEM-PrEP: Adherence patterns and factors associated with adherence to a daily oral study product for pre-exposure prophylaxis. JAIDS. 2014;66:324–31.
Liu A, Glidden DV, Anderson PL, Amico RK, McMahan V, Mehrotra M, et al. Patterns and correlates of PrEP drug detection among MSM and transgender women in the global iPrEx study. JAIDS. 2014;67:528–37.
Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. New Engl J Medicine. 2015;372:509–18.
Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. New Engl J Medicine. 2016;375:2121–32.
van der Straten A, Montgomery ET, Hartmann M, Minnis A. Methodological lessons from clinical trials and the future of microbicide research. Curr HIV-AIDS Rep. 2012;10:89–102.
Amico RK, Marcus JL, McMahan V, Liu A, Koester KA, Goicochea P, et al. Study product adherence measurement in the iPrEx placebo-controlled trial: Concordance with drug detection. JAIDS. 2014;66:530–7.
van der Straten A, Brown ER, Marrazzo JM, Chirenje MZ, Liu K, Gomez K, et al. Divergent adherence estimates with pharmacokinetic and behavioural measures in the MTN-003 (VOICE) study. JIAS. 2016;19:20642.
Baker Z, Javanbakht M, Mierzwa S, Pavel C, Lally M, Zimet G, et al. Predictors of over-reporting HIV pre-exposure prophylaxis (PrEP) adherence among young men who have sex with men (YMSM) in self-reported versus biomarker data. AIDS Behav. 2017;22:1174–83.
MacQueen KM, Tolley EE, Owen DH, Amico RK, Morrow KM, Moench T, et al. An interdisciplinary framework for measuring and supporting adherence in HIV prevention trials of ARV-based vaginal rings. JIAS. 2014;17:19158.
van der Straten A, Montgomery ET, Musara P, Etima J, Naidoo S, Laborde N, et al. Disclosure of pharmacokinetic drug results to understand nonadherence. AIDS. 2015;29:2161–71.
Koester KA, Liu A, Eden C, Amico RK, McMahan V, Goicochea P, et al. Acceptability of drug detection monitoring among participants in an open-label pre-exposure prophylaxis study. AIDS Care. 2015;27:1199–204.
Browne EN, Montgomery ET, Mansfield C, Boeri M, Mange B, Beksinska M, et al. Efficacy is Not Everything: Eliciting Women’s Preferences for a Vaginal HIV prevention product using a discrete-choice experiment. AIDS Behav. 2019;1–9.
Corneli AL, McKenna K, Perry B, Ahmed K, Agot K, Malamatsho F, et al. The science of being a study participant: FEM-PrEP participants’ explanations for overreporting adherence to the study pills and for the whereabouts of unused pills. JAIDS. 2015;68:578–84.
Giguere R, Rael C, Sheinfil A, Balán IC, Brown W, Ho T, et al. Factors supporting and hindering adherence to rectal microbicide gel use with receptive anal intercourse in a phase 2 trial. AIDS Behav. 2018;22:388–401.
Montgomery ET, Mensch B, Musara P, Hartmann M, Woeber K, Etima J, et al. Misreporting of product adherence in the MTN-003/VOICE trial for HIV prevention in Africa: Participants’ explanations for dishonesty. AIDS Behav. 2017;21:481–91.
Musara P, Montgomery ET, Mgodi NM, Woeber K, Akello CA, Hartmann M, et al. How presentation of drug detection results changed reports of product adherence in South Africa. Uganda and Zimbabwe AIDS Behav. 2018;22:877–86.
Balán IC, Giguere R, Brown W, Alex C-D, Horn S, Hendrix CW, et al. Brief participant-centered convergence interviews integrate self-reports, product returns, and pharmacokinetic results to improve adherence measurement in MTN-017. AIDS Behav. 2018;22:986–95.
Levy A, herer A, Zikmund-Fisher BJ, Larkin K, Barnes GD, Fagerlin A. Prevalence of and factors associated with patient nondisclosure of medically relevant information to clinicians. JAMA Netw Open. 2018;1:e185293.
Carroll KM, Farentinos C, Ball SA, Crits-Christoph P, Libby B, Morgenstern J, et al. MET meets the real world: Design issues and clinical strategies in the Clinical Trials Network. JSAT. 2002;23:73–80.
Miller WR, Benefield GR, Tonigan SJ. Enhancing motivation for change in problem drinking: A controlled comparison of two therapist styles. JCCP. 1993;61:455–61.
Miller WR. Motivational Enhancement Therapy Manual. DIANE Publishing. 1995;123.
Miller WR. Enhancing Motivation for Change in Substance Abuse Treatment. DIANE Publishing. 1999;243.
Hester RK, Squires DD, Delaney HD. The Drinker’s Check-up: 12-month outcomes of a controlled clinical trial of a stand-alone software program for problem drinkers. JSAT. 2005;28:159–69.
Amico RK, Miller J, Balthazar C, Serrano P, Brothers J, Zollweg S, et al. Integrated Next Step Counseling (iNSC) for sexual health and PrEP Use among young men who have sex with men: Implementation and observations from ATN110/113. AIDS Behav. 2018;11:1–12.
Amico RK, Mehrotra M, Avelino-Silva VI, McMahan V, Veloso VG, Anderson P, et al. Self-reported recent PrEP dosing and drug detection in an open label PrEP study. AIDS Behav. 2016;20:1535–40.
Amico RK, McMahan V, Goicochea P, Vargas L, Marcus JL, Grant RM, et al. Supporting study product use and accuracy in self-report in the iPrEx study: next step counseling and neutral assessment. AIDS Behav. 2012;16:1243–59.
Moyers TB. The relationship in motivational interviewing. Psychotherapy. 2014;51:358–63.
Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. New Engl J Medicine. 2010;363:2587–99.
Balán IC, Giguere R, Lentz C, Kutner BA, Kajura-Manyindo C, Byogero R, Asiimwe, FB, Makala Y, Jamaya J, Khanyile N, Chetty D, Soto-Torres L, Mayo A, Mgodi NM, Palanee-Phillips T & Baeten J. Client-centered adherence counseling with adherence measurement feedback to support use of the dapivirine ring in MTN-025 (The HOPE Study). (Under review)
IBM SPSS Statistics for Macintosh. 2017.
NVivo qualitative data analysis software. 2019
Spence P, Nel A, van Niekerk N, Derrick T, Wilder S, Devlin B. Post-use assay of vaginal rings (VRs) as a potential measure of clinical trial adherence. J Pharmaceut Biomed. 2016;125:94–100.
Raboud J, Haley L, Montaner J, Murphy C, Januszewska M, Schechter M. Quantification of the variation due to laboratory and physiologic sources in CD4 lymphocyte counts of clinically stable HIV-infected individuals. JAIDS. 1995;10(Suppl 2):S67–73.
Venter W, Chersich MF, Majam M, Akpomiemie G, Arulappan N, Moorhouse M, et al. CD4 cell count variability with repeat testing in South Africa: Should reporting include both absolute counts and ranges of plausible values? Int J STD AIDS. 2018;29:1048–56.
Giguere R, Lentz C, Kajura-Manyindo C, Kutner BA, Dolezal C, Buthelezi M, Lukas I, Nampiira S, Rushwaya C, Sitima E, Katz A, van der Straten A & Balán IC. Building client-centered counseling skills for an HIV-prevention study in Africa. (Under review)
Dimeff L, Baer J, Kivlahan D, Marlatt G. Brief Alcohol Screening and Intervention for College Students (BASICS). New York: Guilford Press; 1999. p. 200.
Riper H, van Straten A, Keuken M, Smit F, Schippers G, Cuijpers P. Curbing problem drinking with personalized-feedback interventions: a meta-analysis. Am J Prev Med. 2009;36:247–55.
Dillard PK, Zuniga J, Holstad MM. An integrative review of the efficacy of motivational interviewing in HIV management. Patient Educ Couns. 2017;100:636–46.
Funding
This study was designed and implemented by the Microbicide Trials Network (MTN), funded by the National Institute of Allergy and Infectious Diseases through individual Grants (UM1AI068633, UM1AI068615 and UM1AI106707) and supported by additional grants through the National Institute of Mental Health (T32MH19139; P30MH43520), all components of the U.S. National Institutes of Health (NIH).
Author information
Authors and Affiliations
Contributions
BAK, RG, CL, CK-M, CD, SB, MG, SN, TN, PM, WM, and ICB performed the research. BAK, RG, CL, CK-M, CD, and ICB designed the research study. BAK, CD, and ICB analysed the data. BAK, RG, CL, CK-M, CD, SB, MG, SN, TN, PM, WM, and ICB wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
I confirm all patient/personal identifiers have been removed or disguised so the patient/person(s) described are not identifiable and cannot be identified through the details of the story.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kutner, B.A., Giguere, R., Lentz, C. et al. Sharing Objective Measures of Adherence to a Vaginal Microbicide Promotes Candor About Actual Use and Bolsters Motivation to Prevent HIV. AIDS Behav 25, 721–731 (2021). https://doi.org/10.1007/s10461-020-03026-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-020-03026-6